
The value of contrast-enhanced ultrasonography to detect the sacroiliac joint for predicting relapse after discontinuation of anti-tumor necrosis factor therapy in patients with ankylosing spondylitis
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease which mainly affects the sacroiliac joints (SIJs), the axial skeleton, and peripheral joints, leading eventually to spinal deformity and stiffness (1). The SIJ is the most frequently involved location presenting symptoms with low back pain (2). The major treatment target is to reach remission or low disease activity in a treat-to-target strategy (3,4). Anti-tumor necrosis factor (TNF) agents have produced significant improvement of signs and symptoms in AS patients in numerous randomized clinical trials (5-7). However, in cases with patients with long periods of remission, severe or repetitive infections, or who are preparing for pregnancy or surgery, discontinuation of anti-TNF treatment may become necessary. Clinical trials indicate that discontinuation of anti-TNF therapy could lead to rapid relapse in almost all AS patients after several weeks or a few months (8-12). However, the literature is unclear as to the reason of relapse. Baraliakos et al. found that spinal inflammation was still existent in AS patients confirmed by MRI after administration of etanercept for 2 years, suggesting spinal inflammation may be the reason for relapse (13). Recurrence may be associated with the latent inflammation in the SIJ, so detecting the inflammation in the SIJ may be helpful in predicting relapse.
To our knowledge, nothing is known about the imaging tools predictive of relapse after discontinuation of anti-TNF therapy in AS patients. The most commonly used imaging techniques in the SIJ are conventional radiography, computed tomography (CT), and magnetic resonance imaging (MRI). Conventional radiography and CT only show structural damages of the SIJ, but cannot detect the existence of inflammation (14). MRI may be helpful in detecting inflammation but is relatively costly and time-consuming (15). Color Doppler ultrasonography (CDUS) allows for detection of abnormal vascularization in the SIJ (16), and plays a certain role in early diagnosis, disease monitoring, and prognosis evaluation (17-19). However, in spite of these promising results, it is sometimes difficult to detect any vascularization of the affected SIJ due to the deep location, small-size vessels, and/or low velocity blood flow. Thus, the use of contrast-enhanced ultrasonography (CEUS) may improve the detection of vascularization of sacroiliitis and help in recognizing the latent inflammation (20,21).
The purpose of this study was to investigate the value of CEUS both in improving the detection of vascularization of sacroiliitis in AS patients in remission and in predicting relapse after drug withdrawal in patients with AS.
Methods
Patient population
This was a prospective observational study. A total of 130 SIJs in 65 patients between January 2014 and October 2018 were examined. This study was approved by the Ethical Committee of Peking University People’s Hospital. All patients gave their written informed consent.
The inclusion criteria were as follows: (I) age ≥18 years old; (II) patients fulfilling the modified New York criteria for the diagnosis of AS (22); (III) continuous and stable treatment with anti-TNFs for the last 6 months; (IV) patients achieving Ankylosing Spondylitis Disease Activity Score (ASDAS) inactive disease criteria (defined as <1.3) after discontinuation of anti-TNF therapy (23).
Exclusion criteria included the following: (I) spinal immobility on clinical examination and bony ankylosis by imaging technique; (II) past or present rheumatoid arthritis and other causes of arthritis; (III) current active tuberculosis or a history of active tuberculosis; (IV) contraindications for the application of SonoVue (Bracco, Milan, Italy) such as hypersensitivity to sulfur hexafluoride; (V) cardiac and pulmonary diseases; and (VI) pregnancy and breastfeeding.
Clinical data at the time of anti-TNF withdrawal were collected, including age, sex, disease duration, HLA-B27, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), erythrocyte sedimentation rate (ESR) (by the Westergren method), and C-reactive protein (CRP) (by nephelometry). All patients had a maximum follow-up of 52 weeks and completed the study when experiencing relapse or maintaining remission of 52 weeks. Relapse was recorded either on telephone visit or outpatient visit at any time if symptoms suggestive of relapse or other problems occurred, and if they did, their clinical symptoms were documented accordingly. Relapse was defined as an increase of two or more items in comparison with the BASDAI at the time of anti-TNF withdrawal (24).
CDUS
An ACUSON S3000 Ultrasound System (Siemens Medical Solutions, Mountain View, CA, USA) and a My Lab 90 Ultrasound System (Esaote Biomedica, Genoa, Italy) with 4–6 MHz convex array transducer were used, operating at a pulse repetition frequency of 1.3 kHz, a color-mode frequency of 2.8 MHz, and low wall filters (125 Hz) which remained fixed throughout the study. The color Doppler gain was optimized by increasing gain until first noise appeared and then reducing gain just enough to suppress the noise. The color box was restricted to the area of the SIJ.
With the patient lying in the prone position, the probe was first oriented transversely at the sacral bone. It was inclined downward to identify the SIJ. The transducer was then turned counterclockwise to reveal the left SIJ and clockwise to reveal the right SIJ. We observed the blood flow in the area of the SIJ by CDUS after each SIJ was visualized. We selected the blood flow signal in the SIJ for the spectral measurement if the blood flow signal could be found both within and around the SIJ. If the arterial blood flow was detected, the resistive index (RI) was measured 3 times, reaching the mean value. Based on our previous research (25), we defined no blood flow, high RI of arterial blood flow (RI ≥0.7), the reversed phase in the diastolic phase or venous blood flow in the bilateral SIJs as negative CDUS; meanwhile, low RI of arterial blood flow (RI <0.7) in the unilateral or bilateral SIJs was defined as positive CDUS.
CEUS
First, a bolus of 1.2 mL agent (SonoVue®) was injected intravenously, and the pipe was immediately washed with 5 mL saline water. At the same time, we started the contrast-enhanced mode for timing and observed the process of blood perfusion in both SIJs continuously and dynamically. After developing of the SIJ, color Doppler and spectral Doppler were used to measure RI. The definitions of positive CEUS and negative CEUS were the same as above.
All the ultrasonographic images, including static and dynamic types, were stored on a disk for each patient. All the examinations were performed by the same ultrasonographer who had more than 5 years of experience in musculoskeletal ultrasound.
Statistical analysis
The Chi-squared test was adopted to compare CDUS with CEUS in AS patients with remission after discontinuation of anti-TNFs. The differences of CEUS for the patients in remission were compared with those for the patients of relapse. A Kaplan-Meier survival analysis was used to calculate the probability of relapse with the duration of remission as survival time and relapse as a binomial covariate for the end point. A Cox proportional hazards regression analysis was exploited to identify possible factors predictive of relapse. P values ≤0.05 were considered statistically significant. In addition, patients were grouped according to the different results of both CDUS and CEUS at the time of discontinuation. The time to relapse in these three groups was compared using a log-rank test.
Results
Table 1 summarizes the demographics and baseline clinical data when anti-TNF treatment was discontinued. Thirteen patients received intravenous infliximab (5 mg/kg/body weight) at 0, 2, and 4 weeks and then every 6 or 8 weeks. The patients received a mean dosage of infliximab 400±75 mg (5 mg/kg) per infusion; 9 patients (69.2%) were treated every 6 weeks and 4 (30.8%) every 8 weeks. Eighteen patients received etanercept 25 mg subcutaneously twice weekly. Thirty-four patients received adalimumab 40 mg subcutaneously every other week. The mean time for the CDUS and CEUS examinations was 5.2±1.3 and 15.5±4.3 minutes respectively. No important clinical side effects of the contrast agent were observed.
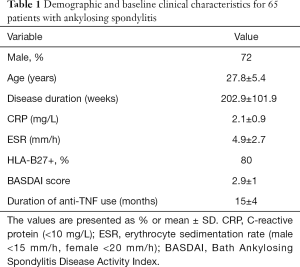
Full table
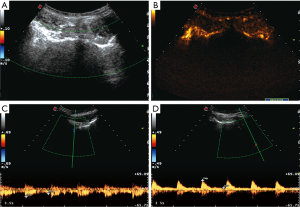
There were three result types of the CDUS and CEUS examinations: positive CDUS and CEUS, negative CDUS and CEUS, and negative CDUS but positive CEUS (Figure 1). After discontinuation of anti-TNF therapy, positive CEUS accounted for 61.5%; this was significantly more than positive CDUS (13.8%) (Table 2). The vascularization detected by CEUS for patients of relapse was significantly different from that of patients with remission (P<0.05). Between the time of withdrawal and relapse, the mean increase of the BASDAI, CRP, and ESR were 3.8 (±1.6), 14.6 mg/L (±21.2 mg/L), and 20.1 mm/h (±28.5 mm/h) respectively. All changes between the time of withdrawal and relapse were statistically significant. The changes in the BASDAI, CRP, and ESR correlated well with the positive CEUS results (P<0.05).

Full table
After discontinuation of anti-TNF therapy, 46 of the 65 patients (70.8%) experienced relapse at the end of the 52-week follow-up. The first two patients (2/65; 3%) came to relapse at the time of 20 weeks; among the 46 patients of relapse, there were 26 between 20 and 30 weeks, 10 between 31 and 40 weeks, 8 between 41 and 50 weeks, and 2 between 51 and 52 weeks. The remaining 19 patients did not relapse (19/65; 29%) until 52 weeks. The mean time to relapse was 31.4 weeks (±8.4 weeks, range 20 to 52), and the median time to relapse was 29 weeks (Figure 2).
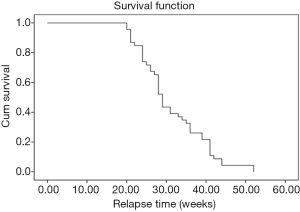
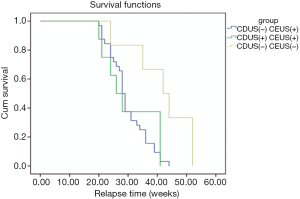
A Kaplan-Meier survival analysis depicted the curve of time to relapse during the follow-up period after discontinuation of anti-TNF therapy in patients with AS (Figure 2). The mean time to relapse was 30.3 weeks for the patients with positive CDUS, and 31.6 weeks for the patients with negative CDUS which was not statistically significant (P=0.723). The mean time to relapse was 29.8 weeks for the patients with positive CEUS and 40 weeks for the patients with negative CEUS. In particular, patients with negative CEUS at the time of discontinuation (n=25) had a longer duration of remission than the patients with positive CEUS (P=0.005) (Figure 3). A Cox proportional hazards regression analysis found that age, gender, HLA-B27, and positive CDUS had no predictive value for relapse, yet positive CEUS (P=0.006) and the disease duration (P=0.049) could predict relapse.
Discussion
The prevalence rate of AS reported is different between countries and ethnicities, but for the Chinese population, the rate is about 0.20–0.54% (26). More than 95% of AS patients have SIJs involved, so the diagnosis of sacroiliitis appears to be very important. The salient features of sacroiliitis are neovascularization, inflammatory infiltration, and intra-articular fluid collection (27). Both ESR and CRP are not very specific in reflecting the inflammation condition (28). MRI might be helpful to detect sacroiliitis through subchondral bone marrow edema (29), but is time-consuming, costly, and limited in patients with contraindications such as metal implants, pacemakers, or claustrophobia. A few previous studies have reported that the vascularization of the SIJ could be revealed by CDUS (17,18). Furthermore, Klauser et al. reported that CEUS had high negative predictive value in the detection of inflamed SIJs for ultrasound contrast agents making the detection of small vessels and slow blood flow possible by increasing the signal-to-noise ratio (20). In our study, we were able to detect more vessels in the SIJ by using CEUS and thus this research supports the thesis put forth by Klauser et al. which states that CEUS can increase the detection of even minor perfusion (20).
Though the advent of anti-TNF agents has produced significant improvement of the patient’s symptoms and signs, a great number of AS patients relapse in a few weeks to a few months after discontinuation of anti-TNF therapy. Even patients with complete remission or reaching ASDAS inactive disease can rapidly relapse. Moreover, there exists little concrete knowledge concerning the clinical parameters predictive of relapse after discontinuation of anti-TNF therapy. Some of the published studies have found that clinical relapse of the disease may be associated with the gradually decreasing or disappearing plasma drug concentration and the emergence of antibodies (30). However, these findings are contradicted by other studies with the opposite conclusions (31). The usefulness of routine antibody testing is controversial as different biological drugs such as infliximab, adalimumab, and etanercept have different immunogenicity and seroconversions (32). Therefore, it is urgent for the clinicians to find an imaging tool to predict relapse in patients with AS after discontinuation of anti-TNF therapy. Deng et al. found that Patient Global Assessment score, CRP, and spinal inflammation could be regarded as predictive factors of relapse in AS patients (33). In our study, we found that the blood flow of the SIJ detected by CEUS after discontinuation of anti-TNF therapy in AS patients seemed to be a predictive factor of relapse.
Despite this, there are still some unsolved questions such as the definitions of remission and relapse in axial spondyloarthritis (34). In this study, we defined remission as ASDAS <1.3 and relapse as a 2-point increase of BASDAI compared with the baseline data. As different definitions of remission and relapse were used, the results acquired may vary. We are looking forward to making a unified standard for the definitions of remission and relapse in the future in order to make disparate research projects in the field more comparable.
The most prominent feature of our study was that we used CEUS to predict relapse of patients with AS in remission after discontinuation of anti-TNF therapy. To the best of our knowledge, there have been no studies that have predicted relapse of AS after discontinuation of anti-TNF therapy through CEUS thus far. The use of CEUS in the early detection of patients in remission could be necessary to some extent to direct the clinician’s choice concerning a retreatment regimen and prevent relapse of the disease. To be honest, although CEUS for the further assessment of the degree of vascularization and for treatment monitoring in joints is an active research field, at present, it cannot be recommended for routine clinical use (35).
All patients enrolled in our study fulfilled the 1984 modified New York criteria for the diagnosis of AS in which radiologic sacroiliitis criterion is an essential prerequisite which makes it possible to detect abnormal ultrasonic manifestation in the SIJ. However, patients with peripheral spondyloarthropathy may have no abnormal imaging manifestation in the SIJ. Therefore, in our future research, we could investigate the ultrasonic manifestations of peripheral arthritis, enthesitis, dactylitis, and synovial and tendon involvement to predict relapse (36-38).
There were several limitations to our study. Firstly, the examinations of CDUS and CEUS were performed by a single sonographer. Secondly, there were no comparisons made with a more standard method like MRI, and this was based on a few reasons. One reason is that some of our patients have a poor economic foundation and cannot afford the expense of MRI examination. Another reason is due to contraindications of MRI examination such as metal implants, pacemakers, or claustrophobia. This is a significant limit in our study. Further investigations are needed to overcome these limitations.
In conclusion, the vascularization in the SIJ could be improved by micro-bubble contrast agents compared with CDUS. CEUS is a safe and highly practical technique for the prediction of relapse in patients with AS after discontinuation of anti-TNF therapy.
Acknowledgments
Funding: This study was supported by funding from the National Natural Science Foundation of China (No. 81171353, 81571684).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethical Committee of Peking University People’s Hospital. All patients gave their written informed consent.
References
- Gibbon WW, Wakefield RJ. Ultrasound in inflammatory disease. Radiol Clin North Am 1999;37:633-51. [Crossref] [PubMed]
- Aggarwal R, Malaviya AN. Clinical characteristics of patients with ankylosing spondylitis in India. Clin Rheumatol 2009;28:1199-205. [Crossref] [PubMed]
- Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H, Dijkmans B, Dougados M, Emery P, Geher P, Hammoudeh M, Inman RD, Jongkees M, Khan MA, Kiltz U, Kvien T, Leirisalo-Repo M, Maksymowych WP, Olivieri I, Pavelka K, Sieper J, Stanislawska-Biernat E, Wendling D, Ozgocmen S, van Drogen C, van Royen B, van der Heijde D. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896-904. [Crossref] [PubMed]
- Smolen JS, Braun J, Dougados M, Emery P, Fitzgerald O, Helliwell P, Kavanaugh A, Kvien TK, Landewé R, Luger T, Mease P, Olivieri I, Reveille J, Ritchlin C, Rudwaleit M, Schoels M, Sieper J. Wit Md, Baraliakos X, Betteridge N, Burgos-Vargas R, Collantes-Estevez E, Deodhar A, Elewaut D, Gossec L, Jongkees M, Maccarone M, Redlich K, van den Bosch F, Wei JC, Winthrop K, van der Heijde D. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6-16. [Crossref] [PubMed]
- van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, Braun J. Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91. [Crossref] [PubMed]
- Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, Wu H, Wang G, Shi Q, Andhivarothai N, Anderson J, Pangan AL. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis 2014;73:587-94. [Crossref] [PubMed]
- Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, Mola EM, Salvarani C, Sanmartí R, Sany J, Sibilia J, Sieper J, van der Linden S, Veys E, Appel AM, Fatenejad S. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-600. [Crossref] [PubMed]
- Baraliakos X, Listing J, Rudwaleit M, Brandt J, Alten R, Burmester G, Gromnica-Ihle E, Haibel H, Schewe S, Schneider M, Sörensen H, Zeidler H, Visvanathan S, Sieper J, Braun J. Safety and efficacy of readministration of infliximab after longterm continuous therapy and withdrawal in patients with ankylosing spondylitis. J Rheumatol 2007;34:510-5. [PubMed]
- Heldmann F, Brandt J, van der Horst-Bruinsma IE, Landewe R, Sieper J, Burmester GR, van den Bosch F, de Vlam K, Geusens P, Gaston H, Schewe S, Appelboom T, Emery P, Dougados M, Leirisalo-Repo M, Breban M, Listing J, Braun J. The European ankylosing spondylitis infliximab cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximab. Clin Exp Rheumatol 2011;29:672-80. [PubMed]
- Chen X, Zhang T, Wang W, Xue J. Analysis of relapse rates and risk factors of tapering or stopping pharmacologic therapies in axial spondyloarthritis patients with sustained remission. Clin Rheumatol 2018;37:1625-32. [Crossref] [PubMed]
- Chen MH, Lee MH, Liao HT, Chen WS, Lai CC, Tsai CY. Health-related quality of life outcomes in patients with rheumatoid arthritis and ankylosing spondylitis after tapering biologic treatment. Clin Rheumatol 2018;37:429-38. [Crossref] [PubMed]
- Sebastian A, Wojtala P, Lubinski L, Mimier M, Chlebicki A, Wiland P. Disease activity in axial spondyloarthritis after discontinuation of TNF inhibitors therapy. Reumatologia 2017;55:157-62. [Crossref] [PubMed]
- Baraliakos X, Brandt J, Listing J, Haibel H, Sorensen H, Rudwaleit M, Sieper J, Braun J. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: clinical and magnetic resonance imaging data. Arthritis Rheum 2005;53:856-63. [Crossref] [PubMed]
- Rudwaleit M, Baraliakos X, Listing J, Brandt J, Sieper J, Braun J. Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis 2005;64:1305-10. [Crossref] [PubMed]
- Braun J, Baraliakos X, Buehring B, Kiltz U, Fruth M. Imaging of axial spondyloarthritis. New aspects and differential diagnoses. Clin Exp Rheumatol 2018;36 Suppl 114:35-42. [PubMed]
- Østergaard M, Eder L, Christiansen SN, Kaeley GS. Imaging in the diagnosis and management of peripheral psoriatic arthritis-The clinical utility of magnetic resonance imaging and ultrasonography. Best Pract Res Clin Rheumatol 2016;30:624-37. [Crossref] [PubMed]
- Arslan H, Sakarya ME, Adak B, Unal O, Sayarlioglu M. Duplex and color Doppler sonographic findings in active sacroiliitis. AJR Am J Roentgenol 1999;173:677-80. [Crossref] [PubMed]
- Unlü E, Pamuk ON, Cakir N. Color and duplex Doppler sonography to detect sacroiliitis and spinal inflammation in ankylosing spondylitis. Can this method reveal response to anti-tumor necrosis factor therapy? The Journal of Rheumatology 2007;34:110-6. [PubMed]
- Zhu J, Xing C, Jiang Y, Hu Y, Hu B, Wang N. Evaluation of complex appearance in vascularity of sacroiliac joint in ankylosing spondylitis by color Doppler ultrasonography. Rheumatol Int 2012;32:69-72. [Crossref] [PubMed]
- Klauser A, Halpern EJ, Frauscher F, Gvozdic D, Duftner C, Springer P, Schirmer M. Inflammatory low back pain: high negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum 2005;53:440-4. [Crossref] [PubMed]
- Klauser AS, De Zordo T, Bellmann-Weiler R, Feuchtner GM, Sailer-Hock M, Sogner P, Gruber J. Feasibility of second-generation ultrasound contrast media in the detection of active sacroiliitis. Arthritis Rheum 2009;61:909-16. [Crossref] [PubMed]
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361-8. [Crossref] [PubMed]
- Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, van der Heijde D. Assessment of SpondyloArthritis international Society. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47-53. [Crossref] [PubMed]
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286-91. [PubMed]
- Hu Y, Zhu J, Xue Q, Wang N, Hu B. Scanning of the sacroiliac joint and entheses by color Doppler ultrasonography in patients with ankylosing spondylitis. J Rheumatol 2011;38:1651-5. [Crossref] [PubMed]
- Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, Le Chen S, Zhang NZ. Rheumatic diseases in China. Arthritis Res Ther 2008;10:R17. [Crossref] [PubMed]
- François RJ, Gardner DL, Degrave EJ, Bywaters EG. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum 2000;43:2011-24. [Crossref] [PubMed]
- Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis 2002;61 Suppl 3:iii8-18. [Crossref] [PubMed]
- Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, Baraliakos X, Bennett A, Braun J, Burgos-Vargas R, Dougados M, Pedersen SJ, Jurik AG, Maksymowych WP, Marzo-Ortega H, Østergaard M, Poddubnyy D, Reijnierse M, van den Bosch F, van der Horst-Bruinsma I, Landewé R. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958-63. [Crossref] [PubMed]
- Aybay C, Ozel S, Aybay C. Demonstration of specific antibodies against infliximab induced during treatment of a patient with ankylosing spondylitis. Rheumatol Int 2006;26:473-80. [Crossref] [PubMed]
- Paramarta JE, Baeten DL. Adalimumab serum levels and antidrug antibodies towards adalimumab in peripheral spondyloarthritis: no association with clinical response to treatment or with disease relapse upon treatment discontinuation. Arthritis Res Ther 2014;16:R160. [Crossref] [PubMed]
- Takase K, Horton SC, Ganesha A, Das S, McHugh A, Emery P, Savic S, Buch MH. What is the utility of routine ANA testing in predicting development of biological DMARD-induced lupus and vasculitis in patients with rheumatoid arthritis? Data from a single-centre cohort. Ann Rheum Dis 2014;73:1695-9. [Crossref] [PubMed]
- Deng X, Zhang J, Zhang J, Huang F. Thalidomide reduces recurrence of ankylosing spondylitis in patients following discontinuation of etanercept. Rheumatol Int 2013;33:1409-13. [Crossref] [PubMed]
- Song IH, Haibel H, Poddubnyy D, Braun J, Sieper J. Withdrawal of biologic therapy in axial spondyloarthritis the experience in early disease. Clin Exp Rheumatol 2013;31:S37-42. [PubMed]
- Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med 2018;39:154-80. [Crossref] [PubMed]
- D'Agostino MA. Enthesitis detection by ultrasound: where are we now? Clin Exp Rheumatol 2018;36 Suppl 114:127-30. [PubMed]
- Spondyloarthropathies Ehrenfeld M. Send to Best Pract Res Clin Rheumatol 2012;26:135-45. [Crossref]
- Riente L, Delle Sedie A, Filippucci E, Iagnocco A, Meenagh G, Grassi W, Valesini G, Bombardieri S. Ultrasound imaging for the rheumatologist IX. Ultrasound imaging in spondyloarthritis. Clin Exp Rheumatol 2007;25:349-53. [PubMed]


