
Evaluating the differences between 1D, 2D, and 3D occupying ratios in reflecting the JOA score in cervical ossification of the posterior longitudinal ligament
Introduction
Ossification of the posterior longitudinal ligament (OPLL) of the cervical spine is a disease of ectopic calcification within the posterior longitudinal ligament, which may cause complicated myelopathy (1,2). The occupying ratio, one of the radiologic static factors, is known to reflect clinical outcome in OPLL. Also, it plays a major role in determining whether to operate anteriorly or posteriorly (3-6). OPLL is a three-dimensional (3D) structure and is also capable of being evaluated in a 3D occupying ratio due to recent developments in imaging technology (7-9). However, since additional conversion programming is required, it is difficult to take 3D measurements rapidly in clinical practice. Conversely, one-dimensional (1D) and two-dimensional (2D) methods are easier to measure because the process is simple. As the most severe segment is the cause of clinical symptoms, we speculated that 2D occupying ratio would reflect better than 1D because it is measured by area. We were also interested to know if the occupying ratio could be applied consistently even if the OPLL is laterally deviated.
The goals of this study were to compare the differences between the 1D length, 2D area, and 3D volume occupying ratios in terms of relationship and clinical outcome and to assess the difference in clinical outcomes and MRI signal intensity between the central and peripheral types of OPLL.
Methods
Patient selection
Between 2012 and 2015, 332 patients with symptomatic OPLL underwent anterior or posterior cervical surgery at our clinic. Among them, all patients with a history of previous cervical surgery, trauma, herniated cervical disc, cervical spondylotic myelopathy, and ossification of the ligamentum flavum were excluded. Cases were only investigated if the cause of the symptoms was only OPLL and not accompanied by any other disease entity. Altogether, 60 patients (49 males and 11 females; mean age of 58.2 years; range, 36–78 years) were included in this study upon applying the exclusion criteria.
Evaluation of OPLL
Clinical symptoms were measured preoperatively by the modified Japanese Orthopedic Association (JOA) score, short-form health survey (SF-36), and neck disability index. The modified JOA score used in our institute has a total of 20 points which quantifies neurological impairment by evaluating upper extremity function (4 points), lower extremity function (4 points), upper/lower extremity and trunk sensibility (each 2 points, total 6 points), and urinary bladder function (6 points) (7).
Institutional board approval was obtained before initiating this study. All OPLL patients who were admitted for surgery underwent routine CT and MRI preoperatively to determine surgical procedure. 1D and 2D occupying ratios were obtained using Centricity PACS (GE Healthcare) (Figure 1). The conventional 1D occupying ratio was defined as the ratio of the maximal ossification thickness to the anteroposterior spinal canal diameter on CT axial imaging. The 2D occupying ratio was defined as the ratio of the area of OPLL to the area of the canal. The 3D model was created with Digital Imaging and Communication in Medicine (DICOM) data from CT images via Centricity PACS, using medical image processing software (MIMICS®, Materialise, Leuven, Belgium). The volume of the OPLL and spinal canal can be measured by confirming the outline of the OPLL and the canal. The 3D occupying ratio was defined as the ratio of the volume of the OPLL to the volume of the canal, marked as 1 cm above and below the most severe level. The measurement process was as follows. First, the spine was divided by the threshold using a function that only contained pixels of images with values greater than or equal to the threshold of a 226 value (7,10). Second, after setting the desired measurement range, the previously specified pixels were erased except for the canal, and the mask was duplicated. Third, on the duplicated mask, only the OPLL was left and the rest was removed. Fourth, the OPLL and spinal canal were reconstructed with 3D rendering. Finally, the volume of the OPLL and spinal canal was calculated automatically based on the processed images.
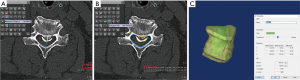
We also evaluated any clinical symptomatic difference depending on the location type of the OPLL. To see if 2D or 3D occupying ratio reflects better than 1D when the OPLL has grown outward from the center of the canal, the axial view of spinal canal on preoperative CT scan was divided vertically into three equal parts. Then, the OPLL was categorized by central type (with the OPLL tip on the middle section in the canal) and peripheral type (with the OPLL tip was both side sections in the canal) according to the location of the most protruded tip of the OPLL in the canal (Figure 2).
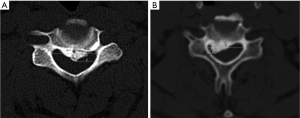
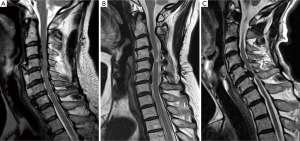
To investigate if there were any differences in intramedullary increased signal intensity (ISI) on T2-weighted MRI depending on the OPLL location type, the ISI was divided into the three following groups as used in another paper by our institute: grade 0, none; grade 1, ISI limited to one disc level; or grade 2, ISI beyond one disc level (4). These grading systems were compared to clinical symptoms, focusing on the size of the ISI on MRI (Figure 3).
Statistical analysis
Statistical analysis was performed using SPSS version 18 for Windows (SPSS, Inc., Chicago, IL, USA). Pearson correlation analysis, independent t-test, and multiple regression analysis were used to analyze the data. A probability value of <0.05 was considered statistically significant.
Results
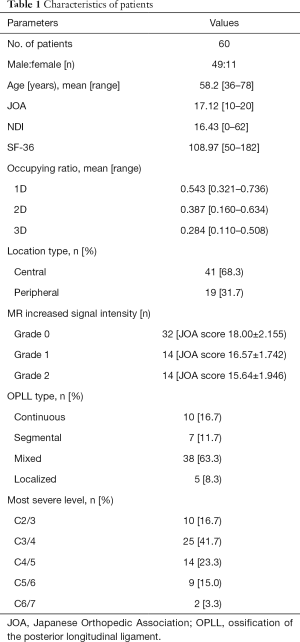
There were four OPLL types including continuous (n=10), segmental (n=7), mixed (n=38), and localized (n=5). According to location type, the number of patients in the central type group and peripheral type group was 41 and 19 respectively. The ISI was observed in 28 of 60 patients using MRI, with the severity distributed as follows: grade 0 in 32, grade 1 in 14, grade 2 in 14 (Table 1). In the correlation analysis, MR ISI showed a significant relationship with 1D, 2D, and 3D occupying ratios (1D: r=0.536, P<0.001; 2D: r=0.525; P=0.001; and 3D: r=0.399; P=0.02, relative).
Full table
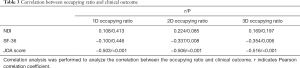
There were close relationships between the 1D and 2D, 1D and 3D, and 2D and 3D occupying ratios (r=0.652, 0.556, 0.776, respectively) (Table 2). Also, the 1D, 2D, and 3D occupying ratios had a significantly negative relationship with the JOA score (1D: r=−0.503, P<0.001; 2D: r=−0.506, P<0.001; and 3D: r=−0.516, P<0.001, respectively) (Table 3). SF 36 showed a result of having a negative relationship with the 2D and 3D but not 1D occupying ratios (1D: r=−0.100, P=0.446; 2D: r=−0.337, P=0.008; and 3D: r=−0.354, P=0.006) (Table 3). However, there were no relationships between the NDI and 1D, 2D, and 3D occupying ratios.

Full table
Full table
There was little difference in the JOA score between the central and peripheral types (17.15 vs. 17.12, t=0.274, P=0.785) (Table 4). The relationship with MR ISI according to the central and peripheral type showed no significant difference (P=0.890).

Full table
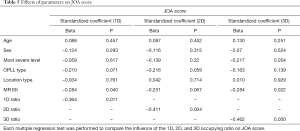
In each multiple regression analysis, the 1D, 2D, and 3D occupying ratios were determined to have more influence on the JOA score (Beta: −0.364, −0.411, −0.462, respectively) than age, sex, most severe level, OPLL type, location type, and MR ISI (Table 5).
Full table
Discussion
The analysis results showed that the 1D, 2D, and 3D occupying ratios have significantly negative relationships with the JOA score. The higher 1D, 2D, and 3D occupying ratios suggest more severe myelopathy in patients with cervical OPLL. However, 1D, 2D, and 3D occupying ratios seem to be highly correlated with each other and there is no difference in the degree of their correlation with the JOA score. Among other clinical outcome parameters, SF-36 showed some correlation, but at a low level. In each multiple regression analysis, the 1D, 2D, and 3D occupying ratios were determined to have more influence on the JOA score than age, sex, most severe level, OPLL type, location type, and MR ISI.
The results showed that the relationship with the JOA score and MRI signal intensity according to location type, whose determination was an additional aim of this study, did not differ, irrespective of the central or peripheral type. This means that even though the OPLL grows outward from the center of the canal, it still reflects well in the 1D occupying ratio, but not with the 2D or 3D methods.
This study aimed to compare the 1D, 2D, and 3D occupying ratios, and included a large number of patients using the 3D method in a single center. As defined in this study for 1D, 2D, and 3D occupying ratios, there are several published papers comparing 1D and 2D occupying ratios or 1D and 3D occupying ratios. Dong et al. published a study that suggested that the 2D occupying ratio may play a significant role in predicting the development of cervical spondylotic myelopathy (11); Lee et al. demonstrated that the 3D occupying ratio shows an independent, significantly negative correlation with the JOA score (7); Wang et al. reported that the 3D occupying ratio is a reliable indicator and better reflected the severity of myelopathy in contrast to the conventional occupying ratio (12).
The above three studies demonstrated that the 2D or 3D occupying ratio is superior to the 1D method for reflecting clinical status such as JOA score. Interestingly, this conclusion differs with the results of our study, where all three occupying ratios reflected the clinical status well. In addition, our study was conducted on 60 patients, which is a number larger than the above three studies’ samples of 36, 34, and 37 patients respectively. Rather, it is estimated that the 1D and 2D methods that have shorter measurement time than 3D will outperform in efficiency when measuring the occupying ratio. The measurement time for the 1D and 2D methods per case is less than 1 minute, while measurements for 3D take more than 15 minutes. Overall, 3D reconstruction processing with DICOM files using the MIMICS software takes more time and effort.
However, the conflicting results of this study in contrast to those from previous studies can be attributable to several factors. First, there could have been a difference in the patient selection group. Moreover, this study was a retrospective study at a single institution. Therefore, there is a possibility that different selection criteria were employed during the patient exclusion process from those of previous studies. Additionally, this study included a total 2-cm section, including 1 cm above and below the most severe level, under the conditions of measuring the 3D occupying ratio, while other studies included the entire cervical OPLL segment. The reason for not including all segments is that the symptoms of patients are affected the most by the most severe level.
Furthermore, a limitation of this study is that dynamic factors, such as the segmental range of motion, which are important factors of myelopathy, were not considered. Matsunaga et al. analyzed 247 patients with OPLL and reported that all of the patients with a space available for the spinal cord (SAC) less than 6 mm suffered myelopathy, whereas the patients with an SAC diameter of 14 mm or greater did not. This indicates that a static occupying factor contributed to the development of myelopathy. However, they also demonstrated that in the patients with myelopathy whose SAC diameter ranged from 6 mm to less than 14 mm, the dynamic factor such as range of motion of the cervical spine was significantly greater (13). In this study, we simply focused on evaluating the correlation between the static factor, the occupying ratio, and the JOA score.
In addition to OPLL, the development of myelopathy can be affected by many other possible factors, such as disc herniation, facet arthropathy, hypertrophy of ligament, ossification of ligamentum flavum, deformity, hypermobility, and instability (14). Thus, in patients with OPLL, other causes may be mixed, resulting in development of myelopathy. This is one of the possible reasons why the occupying ratio does not precisely correlate to the symptoms.
The 3D method can provide new information that has not been previously available (15). In terms of the usability of the 3D method, based on the basic data measured before surgery, the postoperative growth of OPLL can be analyzed (8). Fujimori et al. and Izumi et al. reported that the 3D method of measuring OPLL is very useful to quantitatively evaluate the volume change before or after surgery (16,17). Lee et al. conducted a study on the difference in the growth of OPLL depending on the method of surgery (18). Biomechanical analysis, including the static and dynamic factors, is also available using the 3D finite element model in cervical OPLL (19). Kawaguchi et al. demonstrated that 3D CT is superior to lateral radiography and provided extensive information about OPLL (20,21).
The recent trend in evaluating OPLL has shifted from the conventional method to the 3D method. However, this study shows that although the new technique is a good option, it is not always superior. Sometimes, conventional methods are much easier and comparatively accurate. Conventional methods are also expected to be useful in the future because of their convenience.
Conclusions
In conclusion, all the 1D, 2D, and 3D occupying ratios had significant relationships with the JOA score. There was no difference between the 1D, 2D, and 3D occupying ratios in reflecting the JOA score in cervical OPLL. Also, it is sufficient to reflect the occupying ratio in the clinical outcome without distinguishing between central and peripheral type.
Acknowledgments
This research was performed mainly at the Department of Neurosurgery of the Severance Hospital.
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1288).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional board approval was obtained before initiating this study.
References
- Stapleton CJ, Pham MH, Attenello FJ, Hsieh PC. Ossification of the posterior longitudinal ligament: genetics and pathophysiology. Neurosurg Focus 2011;30:E6. [Crossref] [PubMed]
- Shin JW, Jin SW, Kim SH, Choi JI, Kim BJ, Kim SD, Lim DJ. Predictors of Outcome in Patients with Cervical Spondylotic Myelopathy Undergoing Unilateral Open-Door Laminoplasty. Korean J Spine 2015;12:261-6. [Crossref] [PubMed]
- Iwasaki M. kuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K, Yoshikawa H. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament. Part 1: clinical results and limitations of laminoplasty. Spine 2007;32:647-53. [Crossref] [PubMed]
- Kim B, Yoon DH, Shin HC, Kim KN, Yi S, Shin DA, Ha Y. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J 2015;15:875-84. [Crossref] [PubMed]
- Wu JC, Chen YC, Huang WC. Ossification of the Posterior Longitudinal Ligament in Cervical Spine: Prevalence, Management, and Prognosis. Neurospine 2018;15:33-41. [Crossref] [PubMed]
- Lee SH, Son DW, Lee JS, Kim DH, Sung SK, Lee SW, Song GS. Differences in Cervical Sagittal Alignment Changes in Patients Undergoing Laminoplasty and Anterior Cervical Discectomy and Fusion. Neurospine 2018;15:91-100. [Crossref] [PubMed]
- Lee N, Ji GY, Shin HC, Ha Y, Jang JW, Shin DA. Usefulness of 3-dimensional Measurement of Ossification of the Posterior Longitudinal Ligament (OPLL) in Patients With OPLL-induced Myelopathy. Spine (Phila Pa 1976) 2015;40:1479-86. [Crossref] [PubMed]
- Katsumi K, Watanabe K, Izumi T, Hirano T, Ohashi M, Mizouchi T, Ito T, Endo N. Natural history of the ossification of cervical posterior longitudinal ligament: a three dimensional analysis. Int Orthop 2018;42:835-42. [Crossref] [PubMed]
- Liu H, Li Y, Chen Y, Wu W, Zou D. Cervical curvature, spinal cord MRIT2 signal, and occupying ratio impact surgical approach selection in patients with ossification of the posterior longitudinal ligament. Eur Spine J 2013;22:1480-8. [Crossref] [PubMed]
- Chen D, Chen CH, Tang L, Wang K, Li YZ, Phan K, Wu AM. Three-dimensional reconstructions in spine and screw trajectory simulation on 3D digital images: a step by step approach by using Mimics software. J Spine Surg 2017;3:650-6. [Crossref] [PubMed]
- Dong F, Shen C, Jiang S, Zhang R, Song P, Yu Y, Wang S, Li X, Zhao G, Ding C. Measurement of volume-occupying rate of cervical spinal canal and its role in cervical spondylotic myelopathy. Eur Spine J 2013;22:1152-7. [Crossref] [PubMed]
- Wang Z, Sun Y, Tang Y, Yuan B, Zhou S, Chen X, Jia L. Assessment of Myelopathy in Cervical Ossification of the Posterior Longitudinal Ligament by Magnetic Resonance Imaging-Assisted 3-Dimensional Measurement. World Neurosurg 2018. [PubMed]
- Matsunaga S, Kukita M, Hayashi K, Shinkura R, Koriyama C, Sakou T, Komiya S. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg 2002;96:168-72. [PubMed]
- Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976) 2015;40:E675-93. [Crossref] [PubMed]
- Pakzaban P. A 3-Dimensional-Printed Spine Localizer: Introducing the Concept of Online Dissemination of Novel Surgical Instruments. Neurospine 2018.
- Fujimori T, Iwasaki M, Okuda S, Takenaka S, Kashii M, Kaito T, Yoshikawa H. Long-term results of cervical myelopathy due to ossification of the posterior longitudinal ligament with an occupying ratio of 60% or more. Spine (Phila Pa 1976) 2014;39:58-67. [Crossref] [PubMed]
- Izumi T, Hirano T, Watanabe K, Sano A, Ito T, Endo N. Three-dimensional evaluation of volume change in ossification of the posterior longitudinal ligament of the cervical spine using computed tomography. Eur Spine J 2013;22:2569-74. [Crossref] [PubMed]
- Lee JJ, Shin DA, Yi S, Kim KN, Yoon DH, Shin HC, Ha Y. Effect of posterior instrumented fusion on three-dimensional volumetric growth of cervical ossification of the posterior longitudinal ligament: a multiple regression analysis. Spine J 2018. [PubMed]
- Nishida N, Kanchiku T, Kato Y, Imajo Y, Yoshida Y, Kawano S, Taguchi T. Cervical ossification of the posterior longitudinal ligament: Biomechanical analysis of the influence of static and dynamic factors. J Spinal Cord Med 2015;38:593-8. [Crossref] [PubMed]
- Kawaguchi Y, Urushisaki A, Seki S, Hori T, Asanuma Y, Kimura T. Evaluation of ossification of the posterior longitudinal ligament by three-dimensional computed tomography and magnetic resonance imaging. Spine J 2011;11:927-32. [Crossref] [PubMed]
- Kang MS. W. LJ, Zhang HY, Cho YE, Park YM. Diagnosis of Cervical OPLL in Lateral Radiograph and MRI: Is It Reliable? Korean J Spine 2012;9:205-8. [Crossref] [PubMed]


