
Study on the sub-regions volume of hippocampus and amygdala in schizophrenia
Introduction
Schizophrenia is a chronic mental disorder characterized by distortion of thinking, language, emotion, perception, and self-behavior (1,2). It is generally believed that schizophrenia is related to genetic, environmental, psychosocial factors and social defeat (2,3). In current years, the study found that there was a significant difference in brain immune cell density of patients with schizophrenia compared with healthy people (4), and then the mild encephalitis hypothesis of schizophrenia was put forward (5). The limbic system contains several structures of the brain involving connectivity, architecture, function, and development, including the hippocampus, amygdala, parahippocampal gyrus, internal olfactory region, dentate gyrus, cingulate gyrus and mammillary body (6). Evidence showed that there were changes in limbic system volume in patients with schizophrenia, which may serve as an early indication for the impending development of schizophrenia (7). Both of the hippocampus and amygdala have important positions in the limbic system: hippocampus can store information and is a key part of human learning and memory (8,9), and amygdala has the function of emotional processing (10-12). Sub-regions of the amygdala, including the central-medial and basolateral, are organized at the relative level of emotional processing. Though memory deficits and abnormalities of the amygdala-hippocampal complex have been reported in schizophrenia in the past (13), there was research found no group differences in the amygdala or hippocampal volume between schizophrenia and control (14). Thus, it is necessary to investigate the abnormal changes in hippocampus and amygdala in schizophrenia.
There were longitudinal studies showed that the hippocampus in patients with schizophrenia was vulnerable. Evidence has verified that the volume of the hippocampus was smaller in patients with schizophrenia compared with control (15). Another research showed that hippocampal volume decreased at the initial stage of schizophrenia, and to a lesser extent, this abnormal change occurred in first-degree relatives of schizophrenia patients (16). Furthermore, one study reported that the smaller the volume of the hippocampus, the worse the cognitive ability as well as in schizophrenia (17), which indicated the roles of hippocampus on cognition. Using Freesurfer version 5.1 or 5.2, both Mathew et al. (18) and Haukvik et al. (19) found the volume of CA1, CA2/3, CA4/DG, presubiculum, and subiculum decreased in patients with schizophrenia. Importantly, Iglesias et al. updated the method with a different and more reliable/detailed atlas in Freesurfer 6.0, which included cornus ammonis (CA)1, CA2/3, CA4, molecular layer, alveus, granule cell layer of the dentate gyrus (GC-DG), hippocampal amygdala transition area (HATA), subiculum, presubiculum, parasubiculum, fimbria, hippocampal tail and fissure. Based on the updated method, we make further segmentation on the hippocampus in anticipation of more detailed findings.
Differently, few studies focused on the volume changes of amygdala subregions in patients with schizophrenia, although much research has reported the abnormal volume changes of the whole amygdala (20,21). The previous study has demonstrated that schizophrenia patients have smaller volume in bilateral amygdala and hippocampus compared with healthy control (13). The reduction in the volume of the amygdala was found in patients with early course schizophrenia (22). Another study has found a reduction in somatostatin-immunoreactive (SST-IR) neurons in the amygdala of patients with schizophrenia, which indicated that amygdala change might disrupt anxiety regulation and responses to fear in schizophrenia (23). Many studies have illustrated amygdala atrophy in schizophrenia, while due to the small size of the amygdala, it is difficult to perform sub-regional research in the vivo brain.
Moreover, most of the studies just divided the amygdala into 2–4 sub-regions. For example, Kleinhans et al. (24) divided amygdala into three regions, including centromedial, laterobasal and superficial. Entis et al. (25) divided it into four subregions: basolateral, medial, basal and cortical nucleus. These sub-regions are not fine enough, so detailed division is necessary for research.
In this study, we used the advanced technology to make a more detailed segmentation of hippocampus and amygdala in Freesurfer software on 141 subjects. We hope to identify volume alteration in hippocampus and amygdala in schizophrenia more clearly and provide more reliable results.
Methods
Participants
All participants data were acquired from the Center for Biomedical Research Excellence (COBRE) database (http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html). A total of 141 samples were obtained, including 69 patients with schizophrenia (11 females and 58 males) and 72 control subjects (21 females and 51 males), aged from 18 to 65 years (patients average age =37.739±13.634; control average age =35.857±11.656).
Image acquisition
All data were acquired on the Siemens Trio Tim 3T scanner. The following parameters were collected by the magnetization-prepared rapid gradient echo (MPRAGE) sequence. TE (echo time) =1.64 ms, TR (repetition time) =2,530 ms, FOV (field of view) =256 mm resolution =256×256, 7° flip angle, slice thickness =1.0 mm, Matrix =256×256×176, Voxel size =1×1×1 mm.
Image processing
The reconstructions of the cortical surface were carried out on T1-weighted images in FreeSurfer 6.0 software (http://surfer.nmr.mgh.harvard.edu/). Images were preprocessed by motion correction and brain extraction (26). Talairach transformation and intensity correction were also performed for cortical surface reconstruction. Subsequently, brain tissue was segmented into gray matter, white matter and cerebrospinal fluid. Moreover, the tessellation of the boundary between gray matter and white matter was performed. Subsequently, the segmentation of subcortical structures was examined by a nonlinear warping atlas (27), and the volumes of whole hippocampus and amygdala were obtained. All of the above steps could be implemented using the standard “recon-all” pipeline in FreeSurfer.
Furthermore, the hippocampal/amygdala module, which is only present in the FreeSurfer dev version (
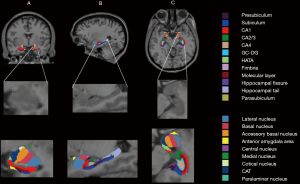
Hippocampus sub-regions segmentation
Thirteen subfields were obtained for hippocampus: cornus ammonis (CA)1, CA2/3, CA4, molecular layer, alveus, GC-DG, HATA, subiculum, presubiculum, parasubiculum, fimbria, hippocampal tail, and fissure.
According to the location and pyramidal layer thickness, subiculum, CA1, CA2, CA3 were defined: the widest region is subiculum, the thinner is CA1, and then is CA2, and CA3 is thinner than CA2. Since the contrast is not clear, the CA2 and CA3 were combined, and because of the thin shape and unreliable division removed alveus volume in this study (29). The presubiculum lies between the subiculum (laterally) and the parasubiculum (medially). CA4 sub-regions locates within the dentate gyrus and fills the inside of the GC-DG label. The GC-DG consists of a polymorphic layer, a molecular layer, and a granule cell layer. The molecular layer lies above the subiculum and underneath the hippocampal fissure. The hippocampal fissure opens medially and extends laterally until there is residual space between the molecular layer of hippocampus and dentate gyrus. The fimbria, which is a white matter structure, lies in the mid-body of the hippocampus. HATA is superior to the other subfield and locates in the median region. More detailed information about the segmentation method could be found in the literature of Iglesias et al. (29).
Amygdala sub-regions segmentation
In total 9 nuclei were segmented for the amygdala, including lateral, basal, accessory-basal, anterior-amygdaloid-area (AAA), central, medial, cortical, corticoamygdaloid-transition (CAT), and paralaminar nucleus. The lateral nucleus is the largest nucleus and the first to appear in the anterior portion of the amygdala. Following the lateral nucleus, the basal nucleus appears. The medial border of the amygdala is CAT, laterally bordering AAA, accessory-basal, basal, paralaminar, and central nucleus along with the anterior-posterior extent. The AAA lies the anterior end of the amygdala, bordering CAT anteriorly and laterally. The central nucleus is between basal nucleus laterally and CAT medially, which appears circular and dasal to accessory basal. The medial nucleus covered nearly all of the lateral-dorsal boundary of CAT. The Cortical nucleus appears as a small circular nucleus, which borders accessary-basal. The paralaminar nucleus borders the basal and lateral nucleus, which is a small, light band. More detailed information about the segmentation method could be found in the literature of Saygin et al. (28).
Statistical analysis
The general linear model (GLM) was performed to analyze the changes in the volume of the hippocampus and amygdala areas in schizophrenia patients compared with healthy people. t-test was performed for statistical analysis of age and handedness, as well as Chi-square test for gender. Regarding the estimated total intracranial volume (eTIV), gender, handedness, and age, we used these variables as covariates for the volume changes between patients and controls. False discovery rate (FDR) correction was performed using Matlab (MathWorks Inc., Natick, MA, USA). Moreover, the significant level was set at 0.05. All steps of imaging processing and statistical were performed by ourselves.
Results
Demographics
Table 1 illustrates the demographic information of each group. There is no significant difference in gender between schizophrenia patients and healthy controls. But age (t=0.389, P=0.046) and handedness (χ2=8.846, P=0.015) show significant differences between two groups.

Full table
Hippocampal volume differences in schizophrenia
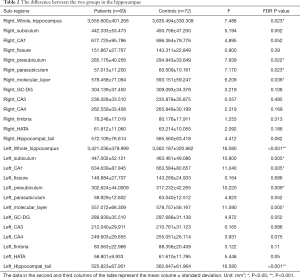
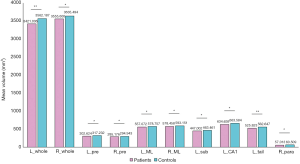
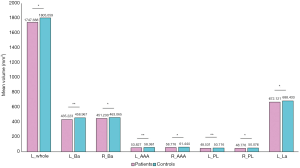
Table 2 shows the statistical analysis of hippocampal sub-regions volumes between two groups. The bilateral hippocampus in patients appear to shrinker volume compared with controls (R: F=7.488, P=0.023; L: F=16.080, P<0.001). When the hippocampus were divided into sub-regions, we found that several areas showed smaller volume in patients, including left hippocampal tail (F=16.580, P<0.001), left subiculum (F=10.800, P=0.005), left CA1 (F=11.040, P=0.005), right parasubiculum (F=7.170, P=0.023), bilateral presubiculum (R: F=7.939, P=0.022; L: F=10.220, P=0.009), and molecular layer (R: F=6.209, P=0.036; L: F=11.590, P=0.005). In other areas of the hippocampus, the difference does not reach a statistically significant level (P>0.05). Figure 2 shows the volume of hippocampal subfields with significant differences in two groups, which indicates the volume of these sub-regions decreased in patients group compared with controls.
Full table
Amygdala volume differences in schizophrenia
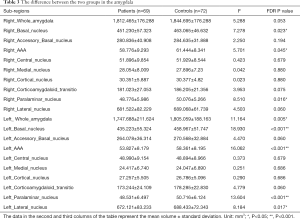
As shown in Table 3, we find a significant reduction in left amygdala volume in schizophrenic patients (F=11.164, P=0.005). Figure 3 describes the raw mean volume of amygdala sub-regions with significant decline in patients, including basal nucleus (R: F=7.278, P=0.023; L: F=18.930, P<0.001), AAA (R: F=5.701, P=0.045; L: F=16.062, P<0.001), paralaminar nucleus (R: F=8.510, P=0.016; L: F=13.604, P<0.001) and left lateral nucleus (F=8.184, P=0.017). In the remaining regions, the difference is not statistically significant (P>0.05).
Full table
Discussion
In this study, we comprehensively compared the volume changes in the subfields of the hippocampus and amygdala between schizophrenia patients and healthy people. The results showed that the whole volume of bilateral hippocampus in patients group was smaller than the control group, as well as several sub-regions including the left hippocampal tail, left subiculum, left CA1, right parasubiculum, bilateral presubiculum and molecular layer. Regarding the amygdala, the main findings were smaller volume in the bilateral basal nucleus, AAA, paralaminar nucleus and left the lateral nucleus, and especially the left side was more significant.
Hippocampal structure
Previous result found in left anterior and midbody of CA1 and CA2, and mid- to anterolateral hippocampal regions showed significant volume changes in schizophrenia patients (31). Also, recently, a review focused on the changes of hippocampal sub-regions in schizophrenia reported that the most prominent volume alteration is CA1 (32). These findings were partly consistent with our results. Perhaps the individual specificity among different dataset and methods resulted in different results. Also, there was evidence showed that CA1 could encode the temporal order of events (33). To a certain degree, this finding indicates that the atrophy of CA1 may lead to thought disorder, which is the symptom of disorganized type. Furthermore, we find that there are more sub-regions on the left hippocampus with volume changes than the right. This is in some way compatible with the hyperactivity of the left hippocampus proposed by Hanlo and his colleagues (34) in schizophrenia. We suppose that patients with schizophrenia due to over-active on the left hippocampus which led to the atrophy in advance of schizophrenia, these hippocampal sub-regions may be more susceptible to disease processes. Besides, there is a pathophysiological process of psychosis discovered by previous researchers. The process involves the increase of synaptic glutamate levels at first, then increase of metabolic demand and blood flow, and down-regulation of hippocampal GABAergic interneurons. Because glutamate receptors exist in the hippocampus, the region may be vulnerable to excitotoxic injury and dysregulation of glutamatergic neurotransmission (35). We speculate whether the volume of specific hippocampal sub-region reduction is related to the number of glutamate receptors.
Volume decrease of these sub-regions is reasonable to some extent in schizophrenia. When we combined the atrophy of hippocampus with schizophrenia subtypes, we found the presubiculum and subiculum shrinking may happen to two types of schizophrenia: paranoid type and catatonic type, because the decreased volumes of these regions have strong associations with bad performance on several cognitive domains (36). Another study reported that parasubiculum was related to movements and the catatonic patients may exhibit purposeless, agitated movement, or maybe almost immobile (37), so the parasubiculum atrophy may be related to the onset of catatonic schizophrenia.
Mathew et al. (18) and Haukvik et al. (19) found CA1, CA2/3, CA4/DG, presubiculum and subiculum appear to shrink in schizophrenia patients. Different from their findings, we only found a volume decrease in the left subiculum and left CA1. The difference maybe due to different segmentation methods and software version we used. Though connections between subiculum and memory retrieval have been proven (38), there was another evidence support left subiculum volume was significantly correlated verbal memory function (39). Based on that, we speculate the volume of left subiculum is more susceptible than the right because it has a stronger relation to memory. To a certain degree, the degree of volume alteration may depend on the stage of schizophrenia, so that volume reduction may occur on the left earlier than the right.
Interestingly, we found that the bilateral molecular layer and left tail also showed significant shrinkage. There was evidence that proved that the molecular layer was related to cognitive processes (40), and the activation of postsynaptic 5-HT(1A) receptors in the hippocampal tail could prevents learned helplessness (41). These findings indicated that schizophrenia patients might have impairments in learning and memory ability.
What is more, in patients with schizophrenia, the extent of hippocampal volume reduction may be related to treatment. The bilateral GC-DG with increased volume before treatment falls to a healthy level after 6 weeks’ treatment, and CA4 reached a close level to a healthy level after treatment (42). This may be due to the high sensitivity of these regions, which will return to normal levels before other hippocampal sub-regions. We did not observe any changes in GC-DG and CA4. Our study did not consider the length of the disease, and the time of treatment and efficacy, this may be one of the reasons why the results differ from previous studies.
Interestingly, there is evidence suggesting that the decreased volume of the hippocampus in patients with schizophrenia is associated with a decrease in the number of cells in the region (43,44). For example, the brain cell density (absolute number of pyramidal cells) of CA1/CA2, CA3, CA4 was significantly decreased, and these sub-regions also showed corresponding volume decrease, which was reported by Falkai et al. (44). These findings suggested the correlation between the volume of the hippocampus and the cell number. However, in our study, we did not perform any work about the cell numbers, and it would be performed in the future.
Amygdala structure
The previous study found the negative connection between stereotypes and the volume of the right amygdala. Moreover, during positive emotional processing, there was a significant negative connection between emotional passivation and left amygdala neural activation. As the condition worsens, the volume of the amygdala begins with one side (left or right) and gradually shrinks to the sides. There was also evidence of a significant increase in dopamine content in the left amygdala in schizophrenia (45). Since the disorder of the dopamine system in schizophrenic patients has been extensively demonstrated (45,46), we speculate that there may be some associations between the disorder of dopamine system and the change of amygdala volume in patients with schizophrenia. In our study, bilateral amygdala volume decreased in the patients’ group but no significance in the right. Combined with these studies, maybe left amygdala is more related to emotion in schizophrenia, rather than the right amygdala. However, Malchow and his colleagues (47) suggest that the recent onset of schizophrenia will also have a decrease in bilateral amygdala volume. Compared with the control group, Okada et al. also found that the amygdala volume of schizophrenic patients showed bilateral volume reduction (13). This difference may be due to the different samples and whether or not the medicine is taken.
Innovatively, we segmented the amygdala into smaller size on schizophrenia patients than the previous study. In elderly schizophrenia patients and controls, previous studies have found structural differences in the medial amygdala, intrauterine-ventral-medial and accessories substrate significant tissue loss, medial, central, and cortical nuclei outer soft tissue loss (48). When compared with psychotic bipolar disorder, the previous study has found volumes of left basolateral and centromedial, and all sub-regions in the right amygdala were decreased in schizophrenia (49). In our study, compared with the control group, basal, AAA, paralaminar nucleus and left lateral nucleus illustrated declined volume in patients group. Based on the previous study, we discussed the associations between the volume decrease of these sub-regions and clinical classification of schizophrenia. The basal nucleus is connected with emotion, cognition, and it can control voluntary motor movements (50,51). AAA is involved in sustaining attention (52) and memory (53), which is occupied by magnocellular cholinergic neurons that secrete acetylcholine (54). So there was research that showed the paralaminar nucleus is related to mood and anxiety disorder (55,56). With the publication of DSM-5, there are five sub-classifications of schizophrenia include paranoid type (delusions or auditory hallucination), disorganized type (thought disorder and flat affect), catatonic type (immobile, or exhibit purposeless, agitated movement), undifferentiated type and residual type (57). It is possible that different subtypes of schizophrenia will have decreased volume of different amygdala sub-regions, such as paranoid type and basal nucleus, disorganized type and paralaminar nucleus. It is undeniable that the reduction in the volume of these sub-regions found in our study may be caused by one certain subtype of schizophrenia, which requires more research to verify.
The different segmentation methods may affect the results. One recent study reported that across development, the connectivity of central, basal, and lateral nucleus continues to change. Development participants did not be included in our study, which is another potential application. Moreover, the functional study on amygdala sub-regions combined with structural changes is necessary to study.
This study has several limitations. First, the sample size is not large, resulting in the existence of sampling errors. Second, the participant included in this study were aged from 18 to 65 years old. There are no younger and order adults. Third, there is the study shows that volume change is related to the course of schizophrenia (58). However, the distinction between the clinical course of the patients was not taken into consideration in our study. Moreover, our data were acquired from the public database, so the pathological subtypes of the schizophrenia were not taken into consideration in this study. Also, other factors that can affect the outcome, lifestyle habits such as smoking and drinking can cause a reduction in the volume of gray matter (59).
In conclusion, this study suggested some new findings on the volume changes of the hippocampus, such as the molecular layer. It is worth emphasizing that we found volume decrease in the basal nucleus, AAA, paralaminar nucleus and left the lateral nucleus in schizophrenia. The decreased volumes in these sub-regions suggest these sub-regions may play more important roles in the study of schizophrenia. We believe there should be more research of this field in the future, especially considering pathological subtypes of schizophrenia.
Acknowledgments
We thank the data providers of the COBRE database.
Funding: This study is funded by the National Key Research and Development Project (2016YFC0103400) and the Fundamental Research Funds for the Central Universities, China (3332018159)., J Qiu was supported by the Taishan Scholars Program of Shandong Province (TS201712065).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull 1987;13:261. [Crossref] [PubMed]
- Selten JG. E. Social defeat: risk factor for schizophrenia? Br J Psychiatry 2005;187:101-2. [Crossref] [PubMed]
- Tsuang MT, Stone WS, Faraone SV. Genes, environment and schizophrenia. Br J Psychiatry Suppl 2001;40:s18-24. [Crossref] [PubMed]
- Bechter K. Relevant News: Mild Neuroinflammation in post-mortem Schizophrenic and Affective Brains: Relevant Idea: Mis- Exaptation possibly involved in Uncontrolled Human Aggression. Neurol Psychiatry Brain Res 2017;23:A1-A.
- Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013;42:71-91. [Crossref] [PubMed]
- Stephani C. Limbic System. In: Daroff R, Aminoff MJ. Encyclopedia of the Neurological Sciences. 2nd edition. Cambridge, Massachusetts: Academic Press, 2014;280:897-900.
- Li X, Black M, Xia S, Zhan C, Bertisch HC, Branch CA, Delisi LE. Subcortical structure alterations impact language processing in individuals with schizophrenia and those at high genetic risk. Schizophr Res 2015;169:76-82. [Crossref] [PubMed]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 2002;116:884-901. [Crossref] [PubMed]
- Hasselmo ME, Wyble BP. Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function. Behav Brain Res 1997;89:1-34. [Crossref] [PubMed]
- Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, Willie JT. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia 2018. Epub ahead of print. [Crossref] [PubMed]
- Gillihan SJ, Rao H, Wang J, Detre JA, Breland J, Sankoorikal GM, Brodkin ES, Farah MJ. Serotonin transporter genotype modulates amygdala activity during mood regulation. Soc Cogn Affect Neurosci 2010;5:1. [Crossref] [PubMed]
- Barbour T, Murphy E, Pruitt P, Eickhoff SB, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res 2010;123:126-36. [Crossref] [PubMed]
- Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, Yasuda Y, Fujimoto M, Watanabe Y, Yahata N. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry 2016;21:1460. [Crossref] [PubMed]
- Killgore WD, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cogn Behav Neurol 2009;22:28-37. [Crossref] [PubMed]
- Hýža M, Kuhn M, Češková E, Ustohal L, Kašpárek T. Hippocampal volume in first-episode schizophrenia and longitudinal course of the illness. World J Biol Psychiatry 2016;17:429-38. [Crossref] [PubMed]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001;11:520-8. [Crossref] [PubMed]
- Jing S, Pearlson GD, Yuhui D, Qingbao Y, Jones TR, Jiayu C, Tianzi J, Juan B, Calhoun VD. In Search of Multimodal Neuroimaging Biomarkers of Cognitive Deficits in Schizophrenia. Biol Psychiatry 2015;78:794-804. [Crossref] [PubMed]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, Clementz B, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry 2014;71:769-77. [Crossref] [PubMed]
- Haukvik UK, Westlye LT, Mørch-Johnsen L, Jørgensen KN, Lange EH, Dale AM, Melle I, Andreassen OA, Agartz I. In Vivo Hippocampal Subfield Volumes in Schizophrenia and Bipolar Disorder. Biol Psychiatry 2015;77:581-8. [Crossref] [PubMed]
- Rijn SV, Aleman A, Swaab H, Kahn RS. Neurobiology of emotion and high risk for schizophrenia: role of the amygdala and the X-chromosome. Neurosci Biobehav Rev 2005;29:385-97. [Crossref] [PubMed]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998;55:433-40. [Crossref] [PubMed]
- Rich AM, Cho YT, Tang Y, Savic A, Krystal JH, Wang F, Xu K, Anticevic A. Amygdala volume is reduced in early course schizophrenia. Psychiatry Res Neuroimaging 2016;250:50-60. [Crossref] [PubMed]
- Pantazopoulos H, Wiseman JT, Markota M, Ehrenfeld L, Berretta S. Decreased Numbers of Somatostatin-Expressing Neurons in the Amygdala of Subjects With Bipolar Disorder or Schizophrenia: Relationship to Circadian Rhythms. Biol Psychiatry 2017;81:536-47. [Crossref] [PubMed]
- Kleinhans NM, Reiter MA, Neuhaus E, Pauley G, Martin N, Dager S, Estes A. Subregional differences in intrinsic amygdala hyperconnectivity and hypoconnectivity in autism spectrum disorder. Autism Res 2016;9:760-72. [Crossref] [PubMed]
- Entis JJ, Doerga P, Barrett LF, Dickerson BC. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. Neuroimage 2012;60:1226-35. [Crossref] [PubMed]
- Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004;22:1060-75. [Crossref] [PubMed]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002;33:341-55. [Crossref] [PubMed]
- Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJ, Boyd E, Reuter M, Stevens A, Van Leemput K, McKee A, Frosch MP, Fischl B, Augustinack JC. Alzheimer's Disease Neuroimaging Initiative. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage 2017;155:370-82. [Crossref] [PubMed]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, Fischl B, Van Leemput K. Alzheimer's Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015;115:117-37. [Crossref] [PubMed]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B. Model-based segmentation of hippocampal subfields in ultra-high resolution in vivo MRI. Med Image Comput Comput Assist Interv 2008;11:235-43.
- Narr KL, Thompson PM, Philip S, Delbert R, Seonah J, Woods RP, Sharon K, Hayashi KM, Dina A, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 2004;21:1563-75. [Crossref] [PubMed]
- Nakahara S, Matsumoto M, van Erp TGM. Hippocampal subregion abnormalities in schizophrenia: A systematic review of structural and physiological imaging studies. Neuropsychopharmacol Rep 2018;38:156-66. [Crossref] [PubMed]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 2004;15:333-51. [Crossref] [PubMed]
- Hanlon FM, Houck JM, Pyeatt CJ, Lundy SL, Euler MJ, Weisend MP, Thoma RJ, Bustillo JR, Miller GA, Tesche CD. Bilateral hippocampal dysfunction in schizophrenia. Neuroimage 2011;58:1158-68. [Crossref] [PubMed]
- Schobel SA, Chaudhury NH, Khan UA, Beatriz P, Styner MA, Iris A, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013;78:81-93. [Crossref] [PubMed]
- Evans TE, Adams HHH, Licher S, Wolters FJ, Lugt AVD, Ikram MK, O'Sullivan MJ, Vernooij MW, Ikram MA. Subregional volumes of the hippocampus in relation to cognitive function and risk of dementia. Neuroimage 2018;178:129-35. [Crossref] [PubMed]
- Riegert C, Galani R, Heilig S, Lazarus C, Cosquer B, Cassel JC. Electrolytic lesions of the ventral subiculum weakly alter spatial memory but potentiate amphetamine-induced locomotion. Behav Brain Res 2004;152:23-34. [PubMed]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci 2005;25:3280-6. [Crossref] [PubMed]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry 1997;63:214-21. [Crossref] [PubMed]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron 2007;54:677-96. [Crossref] [PubMed]
- Joca SR, Padovan CM. Guimarã£Es FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res 2003;978:177-84. [Crossref] [PubMed]
- Li W, Li K, Guan P, Ying C, Xiao Y, Su L, Sweeney JA, Gong Q. Volume alteration of hippocampal subfields in first-episode antipsychotic-naïve schizophrenia patients before and after acute antipsychotic treatment. Neuroimage Clin 2018;20:169-76. [Crossref] [PubMed]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 2002;55:1-10. [Crossref] [PubMed]
- Falkai P, Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986;236:154-61. [Crossref] [PubMed]
- Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature 1983;305:527-9. [Crossref] [PubMed]
- Asada M, Ebihara S, Numachi Y, Okazaki T, Yamanda S, Ikeda K, Yasuda H, Sora I, Arai H. Reduced tumor growth in a mouse model of schizophrenia, lacking the dopamine transporter. Int J Cancer 2008;123:511-8. [Crossref] [PubMed]
- Malchow B, Hasan A, Schneider-Axmann T, et al. Effects of cannabis and familial loading on subcortical brain volumes in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013;263:155-68. [Crossref] [PubMed]
- Prestia A, Boccardi M, Galluzzi S, Cavedo E, Adorni A, Soricelli A, Bonetti M, Geroldi C, Giannakopoulos P, Thompson P, Frisoni G. Hippocampal and amygdalar volume changes in elderly patients with Alzheimer's disease and schizophrenia. Psychiatry Res 2011;192:77-83. [Crossref] [PubMed]
- Mahon PB, Lee DS, Trinh H, et al. Morphometry of the amygdala in schizophrenia and psychotic bipolar disorder. Schizophr Res 2015;164:199-202. [Crossref] [PubMed]
- Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia's role in cognitive coordination. Psychol Rev 2010;117:541-74. [Crossref] [PubMed]
- Weyhenmeyer JA, Gallman EA. Rapid review neuroscience. First edition. Maryland Heights, Missouri: Mosby, 2007.
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res 2000;9:313-25. [Crossref] [PubMed]
- Ridley RM, Bowes PM, Baker HF, Crow TJ. An involvement of acetylcholine in object discrimination learning and memory in the marmoset. Neuropsychologia 1984;22:253-63. [Crossref] [PubMed]
- Gastard M, Jensen SL, Martin JR 3rd, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res 2002;957:207-22. [Crossref] [PubMed]
- deCampo DM, Fudge JL. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev 2012;36:520-35. [Crossref] [PubMed]
- O'Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry 2006;60:479-90. [Crossref] [PubMed]
- Tandon R. Schizophrenia and other psychotic disorders in DSM-5. Clin Schizophr Relat Psychoses 2013;7:16-9. [Crossref] [PubMed]
- Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J, Hong Z, Fischl B, Roffman JL, Zhou J, Sim K, Holt DJ. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry 2017;22:142-52. [Crossref] [PubMed]
- Stone JM, Bhattacharyya S, Barker GJ, McGuire PK. Substance use and regional gray matter volume in individuals at high risk of psychosis. Eur Neuropsychopharmacol 2012;22:114-22. [Crossref] [PubMed]


