
Innovative use of optical coherence tomography catheter via nipple orifice: a case report of first intraductal images of florid ductal hyperplasia
Introduction
The incidence of malignancy in patients with pathologic nipple discharge (PND) varies from 1% to 23% (1). Currently, methylene blue dye or lacrimal probe guided isolated duct excision is still the standard treatment of PND. Majority of those patients are not with cancer-involved ducts and have unnecessary surgery to reveal the definitive histopathology. With conventional imaging, it is not possible to understand whether those ducts involve cancer or not (2). It is well established that ductoscopy is a novel technique that could diagnose a single papilloma; however, it is insufficient to diagnose an in situ or invasive cancer (3,4). Currently, optical coherence tomography (OCT) is used as a non-invasive imaging test that utilizes light waves to capture cross-sectional pictures of retina or coronary arteries (5). In our novel approach, a miniaturized OCT catheter is inserted into the breast ductal system via the nipple orifices to provide detailed real-time imaging of breast ductal epithelial anatomy.
The initial purpose of the study was to understand whether the catheter-based (intraductal) OCT is feasible to insert into the breast ductal system and efficiently scans the ductal epithelium and surroundings. The secondary aim was to determine whether the intraductal OCT could identify early changes or premalignant lesions in the breast duct. If OCT was able to identify precancerous changes in the ducts, this work could open important new channels of research for the early detection of breast cancer.
Case presentation
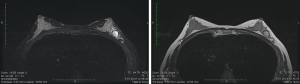
A 49-year-old postmenopausal woman was admitted to our department with a diagnosis of invasive breast cancer achieved by a local clinic presenting with bilateral PND. Physical examination showed a left breast mass at upper outer quadrant with skin and nipple retraction without any palpable lymph nodes in the axilla. Mammogram and ultrasound showed an irregular mass in a high dense breast, with a diameter of 68 mm, which is suspicious, indicating BIRADS 4c. In addition, MRI revealed satellite lesions around the primary tumor, with a diameter of 9 and 7 mm, which was confirmed as malignant with core biopsies. In the ultrasound and MRI, no suspicious lymph nodes were demonstrated (Figure 1). Positron emission tomography showed a left breast mass with SUVmax 16.22 and an axillary lymph node swelling with a SUVmax range of 3.14. No definitive finding of distant metastases was recognized with these modalities. Core biopsy report of the primary tumor showed a grade II invasive ductal carcinoma that did not overexpress the estrogen receptor (ER) or progesterone receptor (PR) and did not express a human epidermal growth factor receptor 2 (HER2), indicating that it was a triple negative tumor. The patient had no known family history of breast or ovarian cancer. Genetic testing revealed c.5265_5266insC (p.Gln1756ProfsTer74) BRCA1 mutation. She was a Caucasian and unaware of any Ashkenazi Jewish heritage. Since she was initially admitted with a locally advanced triple negative invasive cancer, neoadjuvant chemotherapy as carboplatin in addition to weekly paclitaxel, followed by dose-dense doxorubicin and cyclophosphamide was administered. The patient underwent bilateral skin-sparing mastectomy after neoadjuvant chemotherapy and left sentinel lymph node biopsy that revealed negative for cancer cells (0/4) in the frozen section. There were no significant complications; including bleeding, thrombosis or implant loss. Histopathological paraffin evaluation of surgical specimens showed a partial pathological complete response (PCR) with 22 mm residual disease in the breast without any lymph node involvement. Postoperative radiation therapy to her chest-wall and regional lymph node was performed as adjuvant therapy, and she was ambulatory followed without any recurrence at two years follow-up.
This study complied with the Declaration of Helsinki concerning investigations in humans, and the protocol was approved by the Ethics Committee of Acibadem University Medical Center (IRB #766). The patient provided written and informed consent. Patient opted to have a bilateral skin-sparing mastectomy due to bilateral PND and known genetic mutation. We tried to use intraductal OCT for the specimen of the prophylactic right side since the cancer-involved left breast was not suitable to introduce OCT catheter due to nipple retraction.
We decided to evaluate the breast ducts by OCT. Ductoscopy was used to ensure whether the OCT catheter is inside the duct or not. LaDuscope (Polydiagnost, Pfaffenhofen, Germany), which has three channels with a total diameter of 1.1 mm, was used to perform ductoscopy. The SOLEX (Polydiagnost GmbH, Pfaffenhofen, Germany) lumen expander system was used to dilate the ostium of lactiferous ducts (3). While the ductoscopic procedure, the physiologic or pathologic ducts were recorded and the shaft was marked with a pen to calculate the intended depth of the OCT scanning compared to those with pathologic slides. After cannulation, ductoscopy was inserted and confirmed whether the cannulation is successful or the trocar is inside the duct. After safe cannulation, the OCT was inserted through the ductoscopic shaft to launch the scanning. All normal or abnormal images acquired from the scanning were recorded to compare with pathologic slides.
The OCT images were acquired using OCT (C7-XRTM/ILUMIEN, OPTIS, ST. Jude Medical, St. Paul, Minnesota, USA) as previously described (6). Following a Z-offset adjustment, an OCT imaging catheter (DragonflyTM imaging catheter, St. Jude Medical, St. Paul, Minnesota, USA) was positioned so that its imaging lens was distal to abnormal ductal alterations revealed by ductoscopy. All OCT images were obtained using an automatic pullback device with a range of 54 mm and an outer diameter of 0.9 mm (2.7 French) traveling at a rate of 20 mm/s. The OCT had an extreme resolution as 15 µm axially and 25–60 µm laterally. Scan diameter of the device was 10 mm with a maximum frame rate of 100 fps.
As we have been using during ductoscopy, saline was used to infuse directly through the guiding catheter at a rate of 2.5 to 4.5 mL/s using a syringe for enlargement of the duct to facilitate the movement of OCT catheter in the shrunken ex vivo ducts. OCT images were digitally stored for offline analysis. Acquired cross-sectional OCT images were analyzed for the presence of an abnormal lesion or calcification and measured to compare with pathologic slides at that millimeter.
Results
The fluid-yielding duct of the prophylactic mastectomy specimen was scanned with OCT in order to obtain abnormal imaging. Cross-sectional OCT images acquired from the ductal lining showed normal (sub-)epithelial layers proximally and round undefined lesion adjacent to the distended duct distally (Figures 2,3). Ductoscopy failed to show the lesion acquired by OCT. The lesion was 2.5 cm away from the nipple with a diameter of 1 mm, which was measured during OCT scanning. The histopathology revealed a florid ductal hyperplasia (FDH), which causes obliteration in the ductal lumen (Figure 3). This is the first time in the literature an OCT imaging of an FDH in a breast duct was acquired endoscopically or intraductally. The impact of this imaging case was the unique discovery of a high-risk lesion by intraductal OCT, which could not be detected by any conventional imaging.
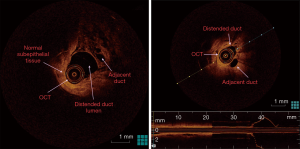
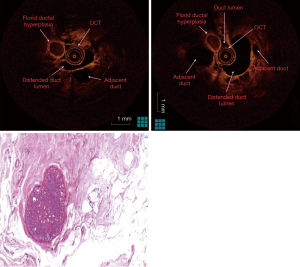
Discussion
To our knowledge, no study has been published in the literature regarding real-time cross-sectional imaging of ductal space by OCT, which enters via nipple orifices. Since the milking ducts are very narrow to enter, only mini-endoscopic instruments such as ductoscopy have been developed to acquire images of pathologic lesions (7). OCT studies on breast include only the analysis of OCT to evaluate surgical margins of lumpectomy specimens (8,9). Furthermore, the OCTs used in those studies did not include a catheter aid to acquire cross-sectional images via natural orifice. However, we used a catheter-based OCT to enter the natural duct orifices in order to achieve direct images from epithelium and layers underneath. Intraductal OCT were able to show ductal epithelium, layers and cavities as cross-sectional images similar to standard hematoxylin & eosin slides. The distention of the duct by saline made the OCT procedure more efficient than the use of the catheter in shrunken ex vivo ducts. The distention did not upgrade resolution, however, made the images of the ductal epithelial layer, lumen, and lesion more clear and distinguishable than the images acquired in shrunken ducts. The use of OCT catheter in vivo in future studies would also need saline infusion and distention from our experience on ductoscopy used for PND which is very similar technically (2-4).
Near-infrared light of OCT enables micron-scale resolution, providing images on the same resolution scale as histopathology. The penetration depth in breast tissue is 1 to 2 mm, making OCT a suitable technology for intraoperative tumor margin assessment (10). In breast cancer surgery, it is essential that borders of the excised specimen do not contain any tumor cells since these positive margins are associated with a higher risk of local recurrence of the primary tumor (11). Invasive ductal adenocarcinomas, which showed infiltrating tumor cells in surrounding tissue and surrounding fibrous tumor stroma, were visible with OCT in several studies (8,9). Furthermore, Nguyen et al. analyzed the resection margins ex vivo with OCT in 37 breast cancer specimens (12). The analysis showed a sensitivity and specificity of 100% and 82%, respectively, in tumor detection compared to histology.
Recently, Yemul et al. (13) assessed mastectomy and breast-conserving surgery specimens from 26 women who were imaged with a handheld OCT probe. Histology slides corresponding to the OCT image region were digitally photographed. OCT and histology images from the same region were paired by selecting the best structural matches. In total, 2,880 OCT images were acquired from 26 breast specimens, and 48 matching OCT-histology image pairs were identified. Differentiation between pathologies was achieved by considering feature boundaries, interior appearance, posterior shadowing or enhancement, and overall morphologic patterns. The results indicate that OCT can be used to identify and distinguish between benign and malignant features in human breast tissue. In our study, round lesion approximately 1mm with a border were found, which caused complete obstruction in the duct shown as hyporeflective interiors and well-circumscribed boundary architecture with distinct edges. These architectural findings of FDH in our study are similar to the findings of Yemul et al. as a hyporeflective interior, less distinct boundaries for hyperplastic ducts.
Since this is the first or only case of a high-risk lesion of breast visualized by real-time OCT, further validation studies needed to determine the sensitivity and specificity of OCT for those lesions. The diameter of the current cardiologic OCT catheter (Dragonfly) was interestingly suitable for the breast ducts; however, the insertion and were a little tough through the ductoscopic trocar, which has a diameter of 1.15 mm. For further studies, the diameter and length of the OCT should be arranged or created according to the diameter of nipple orifice and ductal lining. The OCT catheter had success in showing entire lesion with a diameter of 1mm since the scan range or depth of the OCT is 10 mm.
In conclusion, this is the first case study in literature, which confirms the feasibility of intraductal OCT showing a high-risk lesion in a patient with a BRCA mutation. As a future direction, further studies may confirm whether the patients with a family history or BRCA mutation might be followed by intraductal OCT in addition to conventional imaging. This may help decision making for prophylactic mastectomies in high-risk patients. Furthermore, intraductal OCT may aid ductoscopy to diagnose undefined lesions such as ductal alterations by its high-resolution capability. Integration or combination of ductoscopy and catheter-based OCT may help in the diagnosis of premalignant lesions or in situ ductal cancer to avoid patients with PND from unnecessary isolated duct excisions.
Acknowledgments
The proposed study or innovative idea awarded at Dr. Susan Love Research Foundation’s 8th International Symposium on the Breast is granted by the Susan G. Komen breast cancer organization.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Zielinski J, Jaworski R, Irga-Jaworska N, Pikula M, Hunerbein M, Jaskiewicz J. Use of fiberoductoscopy for the management of patients with pathological nipple discharge: experience of a single center in Poland. Breast Cancer 2018;25:753-8. [Crossref] [PubMed]
- Balci FL, Feldman SM. Exploring breast with therapeutic ductoscopy. Gland Surg 2014;3:136-41. [PubMed]
- Balci FL, Feldman SM. Interventional ductoscopy for pathological nipple discharge. Ann Surg Oncol 2013;20:3352-4. [Crossref] [PubMed]
- Bender O, Balci FL, Yüney E, Akbulut H. Scarless endoscopic papillomectomy of the breast. Onkologie 2009;32:94-8. [PubMed]
- Abdolmanafi A, Duong L, Dahdah N, Adib IR, Cheriet F. Characterization of coronary artery pathological formations from OCT imaging using deep learning. Biomed Opt Express 2018;9:4936-60. [Crossref] [PubMed]
- Ozaki Y, Tanaka A, Nishiguchi T, Komukai K, Taruya A, Satogami K, Kashiwagi M, Kuroi A, Matsuo Y, Ino Y, Kitabata H, Kubo T, Hozumi T, Akasaka T. High-density lipoprotein cholesterol as a therapeutic target for residual risk in patients with acute coronary syndrome. PLoS One 2018;13:e0200383. [Crossref] [PubMed]
- Waaijer L, van Diest PJ, Verkooijen HM, Dijkstra NE, van der Pol CC, Borel Rinkes IH, Witkamp AJ. Interventional ductoscopy in patients with pathological nipple discharge. Br J Surg 2015;102:1639-48. [Crossref] [PubMed]
- Yao X, Gan Y, Chang E, Hibshoosh H, Feldman S, Hendon C. Visualization and tissue classification of human breast cancer images using ultrahigh-resolution OCT. Lasers Surg Med 2017;49:258-69. [Crossref] [PubMed]
- Assayag O, Antoine M, Sigal-Zafrani B, Riben M, Harms F, Burcheri A, Grieve K, Dalimier E, Le Conte de Poly B, Boccara C. Large field, high resolution full-field optical coherence tomography: a pre-clinical study of human breast tissue and cancer assessment. Technol Cancer Res Treat 2014;13:455-68. [PubMed]
- Wang J, Xu Y, Boppart SA. Review of optical coherence tomography in oncology. J Biomed Opt 2017;22:1-23. [PubMed]
- Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol 2009;16:2717-30. [Crossref] [PubMed]
- Nguyen FT, Zysk AM, Chaney EJ, Kotynek JG, Oliphant UJ, Bellafiore FJ, Rowland KM, Johnson PA, Boppart SA. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res 2009;69:8790-6. [Crossref] [PubMed]
- Yemul KS, Zysk AM, Richardson AL, Tangella KV, Jacobs LK. Interpretation of Optical Coherence Tomography Images for Breast Tissue Assessment. Surg Innov 2019;26:50-6. [Crossref] [PubMed]


