
Updates on 18F-FDG-PET/CT as a clinical tool for tuberculosis evaluation and therapeutic monitoring
Introduction
The Mycobacterium tuberculosis (M.tb) is a relatively slow growing complex acid-fast bacillus. M.tb is able to survive in harsh microenvironments within patients in a quiescent state. After exposure to M.tb, an estimated 20–25% of the subjects become infected. One-fourth of the world’s population is latently M.tb-infected and approximately 3–5% of these infected individuals will progress towards developing active tuberculosis (TB) disease during their lifetime (1). Pulmonary disease is present in more than 80% of TB cases; while extrapulmonary TB (EPTB) occurs in about 20% of cases, but can be seen in more than 50% of cases in immunosuppressed populations such as in HIV patients (2). TB can spreads by lymphatic, hematogenous, or direct extension from an infective focus. The presentation of active TB can be very variable, may range from asymptomatic to life threatening as in TB meningitis. Early and accurate diagnosis of TB with early initiation of treatment is important to minimize the morbidity and mortality and to reduce the likelihood of transmission.
Active TB lesions contain activated macrophages and lymphocytes, which have high levels of glucose utilization. 18F-fluorodeoxyglucose (18F-FDG) uptake reflects cell glycolysis and is found in activated macrophages and lymphocytes, both of which are prominent in TB and other granulomatous inflammatory processes, as well as in neoplastic cells. The rate of uptake is reported as a standardized uptake value (SUV), the regional radioactivity concentration divided by the total injected radiotracer dose and adjusted to the patient weight. This creates 18F-FDG signal on positron emission tomography (PET) which forms the basis of 18F-FDG-PET imaging for TB. Lesion activity as determined by 18F-FDG correlates with disease activity. The SUVmax (the maximum standardized uptake value after radiotracer administration) of TB lesions also depend on several factors including host factors such as immune status, comorbid clinical conditions, and M.tb virulence. 18F-FDG-PET scan with/without computed tomography (CT) component can play a very important role in TB patients’ management, and particularly so for those who are sputum negative, unable to produce sputum, or have EPTB (3-5). The lack of specificity is a limitation for 18F-FDG-PET/CT, therefore 18F-FDG-PET/CT scan must be interpreted taking the patient’s clinical information into account. This paper reviews the current understanding of 18F-FDG-PET/CT’s application in TB patients’ management. In this article, for standardization, the term ‘18F-FDG-PET/CT’ is used. However, while 18F-FDG-PET/CT allows the spatial distribution of metabolic activity in the body to be aligned with anatomic image provided by CT, when the CT component is not available, in some cases 18F-PET imaging alone may also provide sufficient information for TB care.
18F-FDG-PET/CT for pulmonary TB
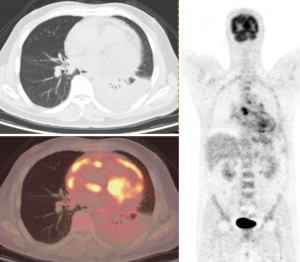
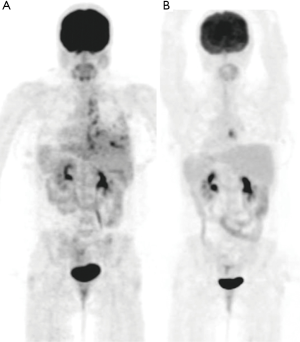
Soussan et al. (6) proposed two patterns of TB based on 18F-FDG-PET appearance, the lung pattern and the lymphatic pattern. The lung pattern relates to restricted infection, patients have predominantly pulmonary symptoms with a parenchymal involvement, with 18F-FDG uptakes on lung consolidation with or without cavitation surrounded by micronodules. Mediastino-hilar lymph nodes can be slightly enlarged with moderate uptake (Figures 1,2). The lymphatic pattern relates to a systemic infection, patients tend to have predominantly systemic and have extra-thoracic involvement, mediastino-hilar lymph nodes can be more enlarged and with higher uptake than in the lung pattern (Figure 3). TB pleural effusions showing 18F-FDG avidity may also be observed and in some instances can be the only manifestation of the disease.
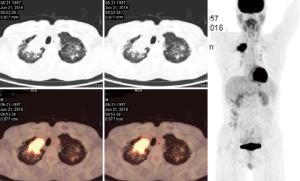
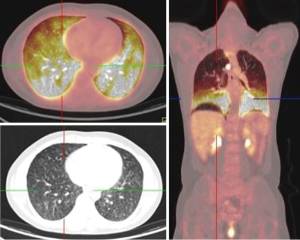
TB cavities are relatively avascular compared with other TB lesions and are more likely to have higher metabolic activity in their walls (Figure 4).
The determination of pulmonary tuberculoma activity may be essential for treatment options, however, diagnosis of active pulmonary tuberculoma is problematic. In this aspect 18F-FDG-PET/CT may play an important role. Kim et al. (7) reported that dual time-point imaging 18F-FDG-PET/CT was able to distinguish active from inactive pulmonary tuberculomas. Active pulmonary tuberculomas had a higher SUVmax at 1 and 2 hours and a greater increase in SUVmax from the early to the late imaging compared with inactive pulmonary tuberculomas, with %ΔSUVmax (percentage change in SUVmax) as a potent predictor for differentiation of tuberculomas’ activity (7).
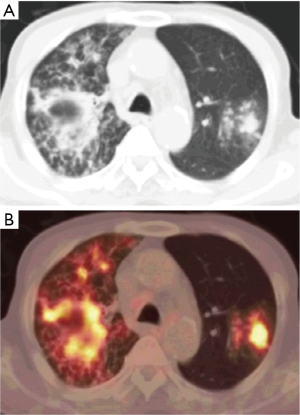
However, 18F-FDG is a nonspecific tracer accumulating in both inflammatory and malignant processes. SUV measurements from both tuberculous and malignant lesions can be high with significant overlap (Figures 5,6). Numerous authors reported 18F-FDG uptake does not reliably distinguish between TB and malignant lesions (8-10). Goo et al. (8) found a mean peak SUV of 4.2±2.2 in pulmonary tuberculomas in 9 of 10 consecutive patients. Sathekge et al. (10) reported a study evaluated the use of dual time-point 18F-FDG-PET/CT, and concluded that SUV measurement was not useful in characterizing lesions as granulomatous or malignant. However, in countries and regions where TB and infections are not endemic, the probability of malignancy in association with positive 18F-FDG-PET findings is high (90% if the patient is older than 60 years); likewise, the probability of malignancy in association with negative 18F-FDG-PET findings is low (<5%) (11).
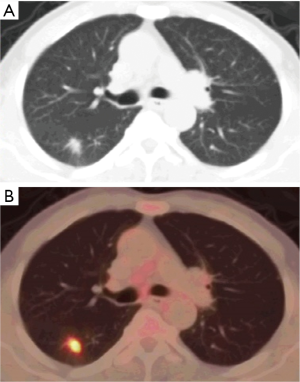
18F-FDG-PET/CT for extrapulmonary TB
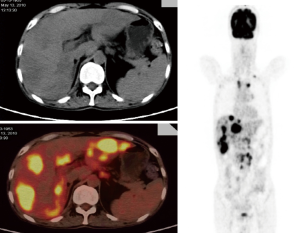
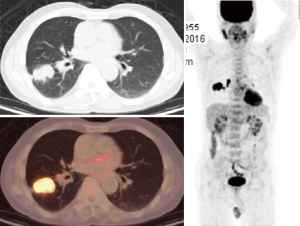
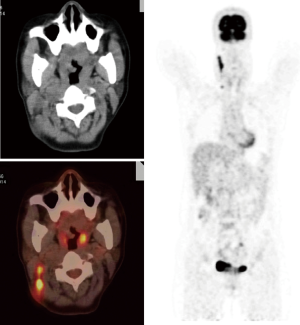
Although pulmonary TB is the most common presentation of the disease, TB can spread to virtually any tissue or organ of the body by haematogenous or lymphatic dissemination or contiguity (Figures 7-9). The most frequently reported EPTB affect sites include lymph nodes, pleura, musculoskeletal, gastrointestinal and genitourinary tract (12-16). The risk of M.tb infection and EPTB increases with advancing immunosuppression. Although definitive diagnosis and exclusion of active TB infection by microbiology and histology is always recommended, 18F-FDG PET/CT may contribute an earlier diagnosis. 18F-FDG PET detects more EPTB lesions than CT. 18F-FDG-PET/CT is particularly helpful when there is multisite involvement, and some sites of the disease may be unsuitable for biopsy such as in the skeleton or the pancreas. Stelzmueller et al. (17) reported more suspicious findings were delineated by 18F-FDG PET than by CT, and the morphological changes as shown by CT did not match with hypermetabolic tissue as shown by 18F-FDG PET in a variety of patients.
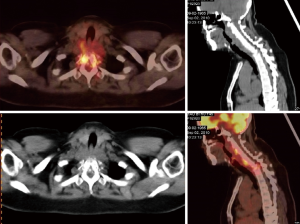
Musculoskeletal TB frequently involves the spine (Figure 7). Failure to identify and treat these areas of involvement in a timely manner may lead to serious complications such as vertebral collapse and spinal compression. 18F-FDG-PET has several potential advantages including a high sensitivity in lesion detection and the fact that image quality is not affected by metal artefacts.
The most common site of EPTB involvement is the lymph nodes, and at least some of them which may be missed on CT scans (Figure 10) (1). However, differentiation of malignant versus TB lymph node involvement is problematic (1). It has also been reported that HIV-related lymphadenopathy may show metabolic uptake that may be difficult to distinguish from TB lymphadenitis (18). Moreover, intense 18F-FDG uptake can be seen in some non-malignant lymphadenopathies such as sarcoidosis, toxoplasmosis and non-specific inflammatory lymphadenopathy.
18F-FDG-PET/CT of subclinical TB infection
TB can be a spectrum of infection states, with transition from latent infection to active disease involving a subclinical phase of disease during which pathology evolves before symptomatic presentation. Autopsy studies in persons died from causes other than TB frequently showed evidence of minimally active disease. Population screening and prevalence surveys also identified asymptomatic patients by either imaging abnormalities consistent with TB or sputum culture positive for M.tb (19). These concepts have been validated by recent animal studies (20,21). In certain individuals a robust acquired immune response may eliminate infection completely while in others a quiescent infection remains with some mycobacteria persisting in non-replicating form (21,22). In a small proportion of patients, the immune response keeps active infection of replicating mycobacteria at the sub-clinical level, while in other patients, active inflammation and TB replication.
The restriction of the bacilli inside granulomas give rise to a latent TB infection (LTBI), defined by no visible symptoms of disease, but dormant and yet alive bacilli in the host. LTBI is not simply a state of bacterial stasis, but rather a state of dynamic bacterial and immunological equilibrium. It has been observed in mouse models that a subpopulation of bacteria continues to replicate, although the size of the bacterial population remains stable (23). In addition, M.tb may accumulate mutations during latency (24). LTBI comprises a reservoir for new disease and ongoing M.tb transmission within communities. Two studies in TB endemic regions of Taiwan and Korea reported that a small percentage of “normal” subjects who underwent whole-body 18F-FDG-PET scanning demonstrated increased 18F-FDG uptake in mediastinal lymph nodes (25,26). LTBI state might last for the entire life span of the individual or progress to active TB by reactivation of the existing infection with a lifetime risk of 5–10% (27). HIV causes a sharp increase in the number of LTBI patients who progress to active disease (3,5).
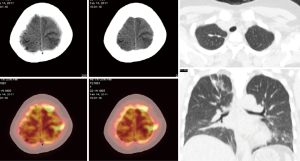
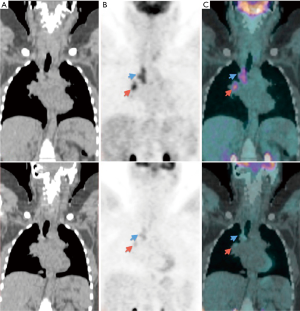
In patients with LTBI, treatment is recommended for persons deemed to be at high risk of developing active disease (28). The preferred treatment is isoniazid daily for 9 months (29). The ability to identify those with LTBI at greatest risk of progression and provide targeted preventive therapy is important. Using 18F-FDG PET/CT scan in 35 asymptomatic, antiretroviral-therapy-naive and HIV-infected adults with latent TB, Esmail et al. (30) identified ten individuals with pulmonary abnormalities suggestive of subclinical active disease who were substantially more likely to progress to clinical disease. These ten subclinical active disease subjects had an initial negative screen for active TB by sputum culture, chest X-ray and symptom screening, while PET/CT showed either infiltrates and/or fibrotic scars or active nodules, and were significantly more likely to have 18F-FDG uptake within mediastinal lymph nodes; whereas the 25 participants with either normal lung parenchyma or discrete small nodules only showed no evidence of subclinical pathology. There were otherwise no significant differences in clinical characteristics between participants who did or did not have evidence of subclinical TB. Moreover, Ghesani et al. (31) reported five cases who were asymptomatic, had normal chest radiographs, and positive QuantiFERON gold assays. Four of the participants had positive 18F-FDG-PET/CT scans involving mediastinal lymph nodes. None of the participants had 18F-FDG uptake in the lungs. None of the 18F-FDG-avid nodes met the radiological criteria for an enlarged lymph node. The patient who had no 18F-FDG uptake had a calcified lung granuloma and calcified hilar nodes noted on CT. Uptake in the mediastinal and hilar lymph nodes regressed during isoniazid prevention therapy (Figure 11) (31).
18F-FDG-PET/CT of clinically cured TB
Currently there is no absolute measure of a sterilizing cure for TB. The current standard treatment period of 6 months was determined by acceptable rates of treatment failure and disease recurrence after discontinuation of chemotherapy (32). M.tb may persist in lung tissue for years after culture negativity has been achieved through anti-TB treatment. Malherbe et al. (33) described an international study involving 113 HIV-negative patients with 18F-FDG-PET/CT scans acquired at different time points before, during, and after anti-TB therapy. On completion of therapy, the study found that patients who had achieved a clinical cure had different patterns of 18F-FDG uptake. In some patients, there was complete resolution of metabolic activity in lesions that were seen at baseline; in others, most of the lesions resolved, with a few just above background or reference structure. In some others, however, some lesions were more intense than the baseline scan or new lesions appeared in patients who achieved and sustained a clinical cure. These dynamic patterns were found after anti-TB treatment and even a year later, regardless of drug sensitivity, sustained culture conversion or clinical cure. The new TB lesions may be due to differential response of the various TB lesions and microevolution in subpopulations of M.tb in patients. These findings present a challenge in interpretation of end-of-treatment 18F-FDG scans. Viable M.tb, with the potential to elicit a host response, often persists even after clinically curative treatment.
Old healed TB usually presents on chest X-ray and CT scan as nodules with fibrotic scarring. These lung lesions often persist long after the end of successful treatment (33-35). It has been noted that latently infected persons who show evidence of fibrotic scarring are up to 15 times more likely to develop disease (36). In a study involving 63 patients with radiological features suggestive of old healed TB lesions, 9 patients had increased 18F-FDG uptake with a SUVmax of 1.5 or more in the old healed lesions (Figure 12) (37). These metabolically active old TB lesions do not necessarily represent active disease, but might reflect an equilibrium between the host’s immune response and the replicating bacilli, and represent an increased risk of development of active TB.
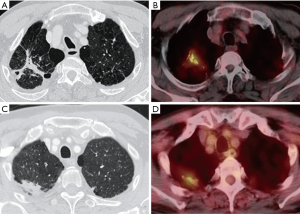
The evidences above suggest that 18F-FDG-PET findings must be carefully correlated with clinical data when interpreting end-of-therapy scans. High uptake of 18F-FDG by PET may both represent ongoing active disease, or simply the host immune system activity that will ultimately prevail.
TB treatment monitoring by 18F-FDG-PET
The slow growth of M.tb necessitates long therapy which renders treatment susceptible to failure due to non-adherence. Standard treatment duration for simple pulmonary or lymph node TB without resistance sign is 6 months according to WHO guidelines, with a relapse rate <5% (38). The treatment duration for EPTB is variable, and shortening treatment is also a major aim of anti-TB drug development. Early response assessment is a key element and particularly relevant for patients with no bacteriological proof and for patients with multi-drug resistant (MDR) or extensively-drug resistant (XDR) TB. Currently, response to treatment in patients with bacillus-positive TB is monitored principally by serial bacteriologic examinations, whereas responses in patients with bacillus-negative TB are usually monitored clinically or radiographically. Up to 20% of patients presenting with pulmonary TB are culture-negative, and fluids for diagnostic analysis are unavailable in most EPTB patients. There is urgent need for biomarkers that allow rapid identification of patients who respond poorly to TB treatment (39,40). 18F-FDG-PET/CT for TB can assess early treatment response when radiological features may remain unchanged, with consequent significant impact on patient management (1,41,42). In a study cohort of 28 subjects with MDR-TB, Chen et al. (43) reported that selected imaging markers can be more sensitive than conventional sputum microbiology in distinguishing successful from unsuccessful treatment. 18F-FDG-PET/CT was the best method for early prediction of treatment results and long term outcome, with 18F-FDG-PET/CT at 2 months demonstrated 96% sensitivity for predicting treatment success and 79% specificity for predicting treatment failure. Similar results were also accomplished by CT, but not until the 6 months scan (43).
Martinez et al. (44) reported 18F-FDG PET/CT was used for therapeutic monitoring in 21 HIV-negative TB patients. They noted that lower SUVmax at month 1 of treatment is a marker for early improvement (Figure 13), and confirms a diagnosis of TB suspected on the basis of histological or clinical symptoms. If the SUVmax is higher on repeated PET/CT, lack of adherence, drug resistance or a misdiagnosis should be considered. Stelzmueller et al. (17) reported that, in a cohort of 35 patients with pulmonary TB or EPTB, persistent 18F-FDG PET/CT lesions in 15 patients and progressive lesions in four patients after a mean treatment duration of 16.1 months; and both components of 18F-FDG PET and CT provided complementary information at initial evaluation and during follow-up. Lefebvre et al. (45) reported that of 18 patients with lymph nodes TB, 18F-FDG PET/CT correctly identified cure in nine patients (no 18F-FDG uptake on posttreatment PET scans) and treatment failure in two patients. However, it should be noted that, due to possible measurement error, a SUVmax variation of <20–25% may not be clinically meaningful (46).
Patients with noncavitary tuberculomas may have no symptoms, and their cultures may be negative. While the majority of pulmonary tuberculomas decrease in size by anti-TB treatment during and even after treatment, a transient enlargement during the early period of treatment can also be observed (47,48). Imaging with 18F-FDG PET/CT may help for these cases. If the lesion shows a decrease in activity, tuberculoma is likely responsive to anti-TB treatment; on the other hand, if 18F-FDG PET/CT demonstrates an increase in lesion activity, then a change of current treatment regimen should be considered.
For TB involvement in the lymph nodes, Sathekge et al. reported two studies where 18F-FDG PET/CT provided prognostic information of distinguishing anti-TB treatment responders from nonresponders (49,50). In 2011, Sathekge et al. (49) reported a study on the relationship between the baseline severity and extent of lymph nodes TB as assessed by 18F-FDG PET and response to treatment at 4 months. There were 24 consecutive HIV patients with newly diagnosed TB who strictly adhered to the treatment regimen, thus treatment failure could be considered a surrogate marker for MDR. It was found that SUVmax of involved lymph node bastions and number of involved lymph node bastions were significantly higher in nonresponders. In 2012, Sathekge et al. (50) further described 20 consecutive HIV patients where 18F-FDG PET/CT scans performed at 4 months after TB treatment as recommended (51). It was found that SUVmax value was significantly higher in nonresponders. Note that FDG uptake by TB-involved lymph nodes of patients with HIV could be significantly higher than the uptake by TB-involved lymph nodes of HIV-negative patients (52), thus a different cut-off value may be applied in TB-infected HIV-negative patients to differentiate nonresponders from responders. Validation studies are also needed to confirm Sathekge et al.’s reports.
Patients with TB-HIV may start on anti-TB therapy and then later followed by antiretroviral therapy. This can cause increased inflammation in existing TB lesions because of immune reconstitution and may be misinterpreted as poor TB treatment response on imaging (3,53). A careful history taking and knowing the time of initiation of antiretroviral therapy are necessary to allow correct interpretation of 18F-FDG-PET/CT images used to monitor anti-TB therapy.
Besides 18F-FDG, other PET tracers have been investigated for imaging of TB, such as 11C-choline, (18F)fluoroethylcholine (18F-FEC), 30-deoxy-30-(18F)fluoro-L-thymidine (18F-FLT), 68Ga-citrate, (18F) sodium fluoride (18FNaF) and radiolabeled anti-TB drugs (5). While some potentially promising results have reported, their clinical application has not been firmly established, and thus beyond the scope of the current review.
Conclusions
18F-FDG-PET/CT not only is an useful clinical tool, it also help to understand the dynamics of pathophysiology and natural course of M.tb infection. 18F-FDG-PET/CT is valuable in TB staging and locating EPTB, identifying patients with subclinical TB, and assessing early treatment response. However, 18F-FDG-PET/CT is costly and involves radiation exposure, it does not need to be recommended to all patients with TB. Though its role in TB management is expanding, guidelines for the use of 18F-FDG-PET/CT in TB clinical management have not been established. Currently the published case series mostly dealt with a few dozens of cases. Validation studies are required for many individual reports (54). There is a strong need for creating and populating global repository of annotated images, where all existing observations, hypotheses and recommendations could be verified, with the help of machine learning, statistics and other data mining techniques (54-57). One up-and-coming project to consider is the TB Portals Program (https://tbportals.niaid.nih.gov/) (55). The current review supports the justification of the TB Portal collection of clinical images as well as clinical information.
Acknowledgments
Funding: This study was partially supported by China TB Portal (OISE-17-63315-1), Grant Assistance Program/10 FOCUS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA, and Sanming Project of Medicine in Shenzhen (No. SZSM201611030), China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization: Tuberculosis. Available online: . Accessed May 22, 2019.https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Ramírez-Lapausa M, Menendez-Saldana A, Noguerado-Asensio A. Extrapulmonary tuberculosis: an overview. Rev Esp Sanid Penit 2015;17:3-11. [Crossref] [PubMed]
- Ankrah AO, Glaudemans AWJM, Maes A, Van de Wiele C, Dierckx RAJO, Vorster M, Sathekge MM. Tuberculosis. Semin Nucl Med 2018;48:108-30. [Crossref] [PubMed]
- Vorster M, Sathekge MM, Bomanji J. Advances in imaging of tuberculosis: the role of 18F-FDG PET and PET/CT. Curr Opin Pulm Med 2014;20:287-93. [Crossref] [PubMed]
- Ankrah AO, van der Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW. PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging 2016;4:131-44. [Crossref] [PubMed]
- Soussan M, Brillet PY, Mekinian A, Khafagy A, Nicolas P, Vessieres A, Brauner M. Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur J Radiol 2012;81:2872-6. [Crossref] [PubMed]
- Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, Nam HY, Kim JS. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging 2008;35:808-14. [Crossref] [PubMed]
- Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, Chung JK. Pulmonary tuberculoma evaluated by means of FDG PET: Findings in 10 cases. Radiology 2000;216:117-21. [Crossref] [PubMed]
- Sathekge M, Maes A, Kgomo M, Stoltz A, Pottel H, Van de Wiele C. Impact of FDG PET on the management of TBC treatment. A pilot study. Nuklearmedizin 2010;49:35-40. [Crossref] [PubMed]
- Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J 2010;100:598-601. [Crossref] [PubMed]
- Winer-Muram HT. The solitary pulmonary nodule. Radiology 2006;239:34-49. [Crossref] [PubMed]
- Batún-Garrido JA, Salas-Magaña M, García-Padrón OA, Valencia-Serrano N. Two cases of tuberculous spondylodiscitis: a rare manifestation of extrapulmonary tuberculosis. Quant Imaging Med Surg 2017;7:132-7. [Crossref] [PubMed]
- Arbault A, Ornetti P, Loffroy R, Chevallier O, Avril J, Pottecher P. Parascapular mass revealing primary tuberculosis of the posterior arch. Quant Imaging Med Surg 2016;6:454-6. [Crossref] [PubMed]
- Wang YX, Chen CR, He GX, Tang AR. CT findings of adrenal glands in patients with tuberculous Addison's disease. J Belge Radiol 1998;81:226-8. [PubMed]
- Wang Y, He G, Zhan W, Jiang H, Wu D, Wang D, Tang A. CT findings in splenic tuberculosis. J Belge Radiol 1998;81:90-1. [PubMed]
- Gambhir S, Ravina M, Rangan K, Dixit M, Barai S, Bomanji J. International Atomic Energy Agency Extra-pulmonary TB Consortium. Imaging in extrapulmonary tuberculosis. Int J Infect Dis 2017;56:237-47. [Crossref] [PubMed]
- Stelzmueller I, Huber H, Wunn R, Hodolic M, Mandl M, Lamprecht B, Schinko H, Fellner F, Skanjeti A, Giammarile F, Colletti PM, Rubello D, Gabriel M. 18F-FDG PET/CT in the Initial Assessment and for Follow-up in Patients With Tuberculosis. Clin Nucl Med 2016;41:e187-94. [Crossref] [PubMed]
- Ankrah AO, Glaudemans AW, Klein HC, Dierckx RAJO, Sathekge M. The role of nuclear medicine in the staging and management of human immune deficiency virus infection and associated diseases. Nucl Med Mol Imaging 2017;51:127-39. [Crossref] [PubMed]
- Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. Latent tuberculosis infection--Revisiting and revising concepts. Tuberculosis (Edinb) 2015;95:373-84. [Crossref] [PubMed]
- Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009;7:845-55. [Crossref] [PubMed]
- Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol 2010;185:15-22. [Crossref] [PubMed]
- Via LE, Schimel D, Weiner DM, Dartois V, Dayao E, Cai Y, Yoon YS, Dreher MR, Kastenmayer RJ, Laymon CM, Carny JE, Flynn JL, Herscovitch P, Barry CE 3rd. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18 F]2-Fluoro- Deoxy-D-Glucose Positron Emission Tomography and Computed Tomography. Antimicrob Agents Chemother 2012;56:4391. [Crossref] [PubMed]
- Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med 2009;15:211-4. [Crossref] [PubMed]
- Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, Flynn JL, Fortune SM. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet 2011;43:482-6. [Crossref] [PubMed]
- Kwan A, Seltzer M, Czernin J, Chou MJ, Kao CH. Characterization of hilar lymph node by 18F-fluoro-2-deoxyglucose positron emission tomography in healthy subjects. Anticancer Res 2001;21:701e6.
- Kim DW, Kim CG. Dual-time point positron emission tomography findings of benign mediastinal lymph nodes in a tuberculosis-endemic region. Jpn J Radiol 2011;29:682-7. [Crossref] [PubMed]
- Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Rev Mol Med 2011;13:e39. [Crossref] [PubMed]
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR Jr, Chaisson RE. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365:2155-66. [Crossref] [PubMed]
- Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. Available online: . Accessed May 22, 2019https://www.cdc.gov/tb/publications/ltbi/treatment.htm
- Esmail H, Lai RP, Lesosky M, Wilkinson KA, Graham CM, Coussens AK, Oni T, Warwick JM, Said-Hartley Q, Koegelenberg CF, Walzl G, Flynn JL, Young DB, Barry Iii CE, O'Garra A, Wilkinson RJ. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[18F]fluoro-D-glucose positron emission and computed tomography. Nat Med 2016;22:1090-3. [Crossref] [PubMed]
- Ghesani N, Patrawalla A, Lardizabal A, Salgame P, Fennelly KP. Increased cellular activity in thoracic lymph nodes in early human latent tuberculosis infection. Am J Respir Crit Care Med 2014;189:748-50. [Crossref] [PubMed]
- Cox HS, Morrow M, Deutschmann PW. Long term efficacy of DOTS regimens for tuberculosis: systematic review. BMJ 2008;336:484-7. [Crossref] [PubMed]
- Malherbe ST, Shenai S, Ronacher K, Loxton AG, Dolganov G, Kriel M, Van T, Chen RY, Warwick J, Via LE, Song T, Lee M, Schoolnik G, Tromp G, Alland D, Barry CE 3rd, Winter J, Walzl J. the Catalysis TB-Biomarker Consortium. Persisting PET-CT lesion activity and M. tuberculosis mRNA after pulmonary tuberculosis cure. Nat Med 2016;22:1094-100. [Crossref] [PubMed]
- Seon HJ, Kim YI, Lim SC, Kim YH, Kwon YS. Clinical significance of residual lesions in chest computed tomography after anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis 2014;18:341-6. [Crossref] [PubMed]
- Heysell SK, Thomas TA, Sifri CD, Rehm PK, Houpt ER. 18-fluorodeoxyglucose positron emission tomography for tuberculosis diagnosis and management: A case series. BMC Pulm Med 2013;13:14. [Crossref] [PubMed]
- Steinbrück P, Dănkovã D, Edwards LB, Doster B, Livesay VT. Tuberculosis risk in persons with "fibrotic" x-ray lesions. Bull Int Union Tuberc 1972;47:135-59. [PubMed]
- Jeong YJ, Paeng JC, Nam HY, Lee JS, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. (18)F-FDG positron-emission tomography/computed tomography findings of radiographic lesions suggesting old healed tuberculosis. J Korean Med Sci 2014;29:386-91. [Crossref] [PubMed]
- World Health Organization. Treatment of tuberculosis guidelines, Fourth Edition. Available online: . Accessed October 11, 2015.https://www.who.int/tb/publications/2010/9789241547833/en/
- Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis 2010;14:131-40. [PubMed]
- Wáng YX, Chung MJ, Skrahin A, Rosenthal A, Gabrielian A, Tartakovsky M. Radiological signs associated with pulmonary multi-drug resistant tuberculosis: an analysis of published evidences. Quant Imaging Med Surg 2018;8:161-73. [Crossref] [PubMed]
- Sathekge MM, Ankrah AO, Lawal I, Vorster M. Monitoring Response to Therapy. Semin Nucl Med 2018;48:166-81. [Crossref] [PubMed]
- Coleman MT, Chen RY, Lee M, Lin PL, Dodd LE, Maiello P, Via LE, Kim Y, Marriner G, Dartois V, Scanga C, Janssen C, Wang J, Klein E, Cho SN, Barry CE 3rd, Flynn JL. PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med 2014;6:265ra167. [Crossref] [PubMed]
- Chen RY, Dodd LE, Lee M, Paripati P, Hammoud DA, Mountz JM, Jeon D, Zia N, Zahiri H, Coleman MT, Carroll MW, Lee JD, Jeong YJ, Herscovitch P, Lahouar S, Tartakovsky M, Rosenthal A, Somaiyya S, Lee S, Goldfeder LC, Cai Y, Via LE, Park SK, Cho SN, Barry CE 3rd. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med 2014;6:265ra166. [Crossref] [PubMed]
- Martinez V, Castilla-Lievre MA, Guillet-Caruba C, Grenier G, Fior R, Desarnaud S, Doucet-Populaire F, Boué F. (18)F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis 2012;16:1180-5. [Crossref] [PubMed]
- Lefebvre N, Argemi X, Meyer N, Mootien J, Douiri N, Sferrazza-Mandala S, Schramm F, Weingertner N, Christmann D, Hansmann Y, Imperiale A. Clinical usefulness of 18F-FDG PET/CT for initial staging and assessment of treatment efficacy in patients with lymph node tuberculosis. Nucl Med Biol 2017;50:17-24. [Crossref] [PubMed]
- Wahl R L, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50:122S-50S. [Crossref] [PubMed]
- Lee HS, Oh JY, Lee JH, Yoo CG, Lee CT, Kim YW, Han SK, Shim YS, Yim JJ. Response of pulmonary tuberculomas to anti-tuberculous treatment. Eur Respir J 2004;23:452-5. [Crossref] [PubMed]
- Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med 2008;33:1-3. [Crossref] [PubMed]
- Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med 2011;52:880-5. [Crossref] [PubMed]
- Sathekge M, Maes A, D'Asseler Y, Vorster M, Gongxeka H, Van de Wiele C. Tuberculous lymphadenitis: FDG PET and CT findings in responsive and nonresponsive disease. Eur J Nucl Med Mol Imaging 2012;39:1184-90. [Crossref] [PubMed]
- American Thoracic Society. CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep 2003;52:1-77.
- Sathekge M, Maes A, Kgomo M, Pottel H, Stolz A, Van De Wiele C. FDG uptake in lymph-nodes of HIV+ and tuberculosis patients: implications for cancer staging. Q J Nucl Med Mol Imaging 2010;54:698-703. [PubMed]
- French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 2009;48:101-7. [Crossref] [PubMed]
- Sjölander H, Strømsnes T, Gerke O, Stroobants S. Value of FDG-PET/CT for treatment response in tuberculosis: a systematic review and meta-analysis. Clin Transl Imaging 2018;6:19-29. [Crossref]
- Rosenthal A, Gabrielian A, Engle E, Hurt DE, Alexandru S, Crudu V, Sergueev E, Kirichenko V, Lapitskii V, Snezhko E, Kovalev V, Astrovko A, Skrahina A, Taaffe J, Harris M, Long A, Wollenberg K, Akhundova I, Ismayilova S, Skrahin A, Mammadbayov E, Gadirova H, Abuzarov R, Seyfaddinova M, Avaliani Z, Strambu I, Zaharia D, Muntean A, Ghita E, Bogdan M, Mindru R, Spinu V, Sora A, Ene C, Vashakidze S, Shubladze N, Nanava U, Tuzikov A, Tartakovsky M. The TB Portals: an Open-Access, Web-Based Platform for Global Drug-Resistant-Tuberculosis Data Sharing and Analysis. J Clin Microbiol 2017;55:3267-82. [Crossref] [PubMed]
- Teramoto A, Fujita H, Yamamuro O, Tamaki T. Automated detection of pulmonary nodules in PET/CT images: Ensemble false-positive reduction using a convolutional neural network technique. Med Phys 2016;43:2821-7. [Crossref] [PubMed]
- Lakhani P, Sundaram B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017;284:574-82. [Crossref] [PubMed]


