Iatrogenic arteriovenous fistula of the iliac artery after lumbar discectomy surgery: a systematic review of the last 18 years
Introduction
An abnormal connection between an artery and a vein is defined as an arteriovenous fistula (AVF) (1). Accordingly, an iliac arteriovenous fistula (IAVF) is formed when there is an abnormal connection between an iliac artery [for instance, a common iliac artery (CIA), an internal iliac artery (IIA), or an external iliac artery (EIA)] and a vein [for instance, a common iliac vein (CIV), an internal iliac vein (IIV), an external iliac vein (EIV), or an inferior vena cava (IVC)] (2). Depending on the pathogenesis, IAVFs can be divided into two types: congenital and acquired types. A congenital IAVF is mainly caused by the congenital dysplasia of an iliac artery and a vein with subsequent formation of an IAVF (3). Meanwhile, an acquired IAVF is mainly caused by trauma, especially an iatrogenic injury (1,2).
An IAVF after a lumbar discectomy surgery (LDS) is a rare occurrence. The aim of this study was to retrospectively analyze the clinical data of a patient with IAVF after an LDS, performed in our hospital, dating between the years 2000 to 2018. In addition to that data, literature from the databases about IAVFs after LDSs were also retrieved with emphasis placed upon the patients’ clinical data.
The institutional review board of our hospital agreed to this present study. This being a retrospective study, any the informed consent requirement from the patient was waived.
Material and methods
Case presentation
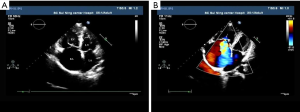
A 47-year-old woman was admitted with symptoms of exertional dyspnea, bilateral lower extremity edema, abdominal distention, and anorexia for more than nine months. She was diagnosed with primary pulmonary hypertension (PPH) at two other hospitals. Cardiac and pulmonary catheterizations were performed revealing increased pressure in her right heart and pulmonary arteries. Echocardiography showed an enlarged right atrium and ventricle, severe tricuspid insufficiency, and increased right ventricular systolic pressure (Figure 1A,B). After symptomatic treatments were completed, her conditions improved.
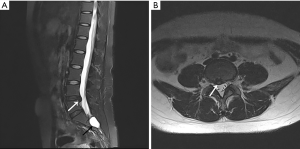
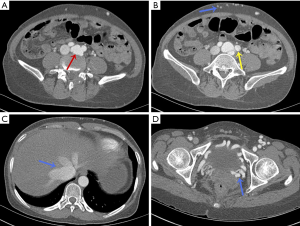
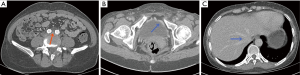
Nevertheless, the patient’s symptoms deteriorated, and this prompted her to visit our hospital. Physical examination findings included raised jugular venous pulse (JVP), positive hepato-jugular reflux, bilateral lower extremities edema, grade III-VI ejection systolic murmur, pulsatile mass with thrill, and a continuous bruit over her left lower abdominal quadrant. Medical history revealed an L4–L5 level LDS 9 months previous (Figure 2A,B). With a provisional diagnosis of iatrogenic vascular injury, we performed computed tomography angiography (CTA) of the thoracic aorta (TA) and abdominal aorta (AA) (Figure 3A,B,C,D) which showed AVF and pseudo aneurysm formation between the left CIA and CIV (A, red arrow), with dissection of the left CIA (B, yellow arrow). Dilated vessels and early opacification of hepatic, IVC, pelvic, and subcutaneous veins of the anterior-inferior abdominal wall were also noted at the arterial phase CT scan (B,C,D, blue arrow).
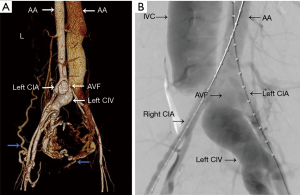
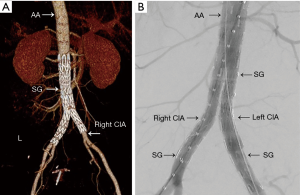
The patient underwent endovascular repair surgery with 2 covered stents (24 mm × 120 mm and 14 mm × 100 mm, XJZDF-24120 and XJZDZ-24040 respectively, ANKURA, LifeTech Scientific Corporation, Shenzhen City, Guangdong, China). CTA after repair (Figure 4A,B,C) demonstrated complete resolution of AVF, pseudo-aneurysm, dissection of the left CIA (A, red arrow), opacification of veins during venous phase (B,C, blue arrow), and absence of dilated vessels. CTA with volume rendering (VR) post-processing technology and digital subtraction angiography (DSA) (Figures 5,6) of the TA and AA before and after the surgery showed findings identical to those seen on Figures 3 and 4, respectively. She was discharged 1 week after the surgery with the advice of a monthly follow-up. During her 2-year period of follow-up, she was found to be doing well without any discomfort.
Literature search
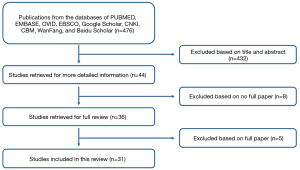
Two reviewers comprehensively searched every potential publication in English on PUBMED, EMBASE, OVID, EBSCO, and Google Scholar, along with publications in Chinese on the China National Knowledge Infrastructure (CNKI), Chinese Biomedical (CBM), WanFang, and Baidu Scholar databases (Figure 7). Publication dates were restricted from January 2000 to October 2018, and papers employing posterior surgical approaches were included while anterior surgical approaches were excluded. If there was any disagreement, it was settled through discussion and consensus.
The following literature retrieval strategy was used separately or in combination with the PUBMED database:
- (“Disc” OR “Lumbar disc” OR “Lumbar intervertebral disc” OR “Disc herniation” OR “Lumbar disc herniation” OR “Lumbar intervertebral disc herniation” OR “Disc prolapse” OR “Lumbar disc prolapse” OR “Lumbar intervertebral disc prolapse” OR “Disc surgery” OR “Lumbar disc surgery” OR “Disc herniation surgery” OR “Lumbar disc herniation surgery” OR “Lumbar intervertebral disc surgery” OR “Disc prolapse surgery” OR “Lumbar discectomy” OR “Surgery” OR “Discectomy”);
- (“Iatrogenic” OR “Iatrogenic trauma” OR “Iatrogenic injury” OR “Vascular injury” OR “Vascular trauma” OR “Iatrogenic vascular trauma” OR “Iatrogenic vascular injury” OR “Perforation” OR “Laceration” OR “Fistula” OR “Arteriovenous fistula” OR “Pseudoaneurysm” OR “Dissection” OR “Trauma” OR “Injury”);
- (“Lumbar” OR “Spine” OR “Spinal”);
- (I) AND (II) AND (III).
The reference list from the retrieved papers was also crosschecked for overlooked articles. Language of the publications was not restricted, but the publication date was restricted to between January 2000 and October 2018. Choice of this time window was based on the following two reasons: (I) there was a review on this topic published in 2002 by Papadoulas et al. (1). In that paper, the authors gave a general review of vascular injury complications of LDS (including laceration, AVF, and pseudo aneurysm), but the complication of IAVF after an LDS was not comprehensively or specifically treated. Thus, the authors of this present study wanted to update that 2002 article with emphasis placed upon the complication of IAVF only; (II) the increasing application of endovascular repair technology for the treatment of IAVF after an LDS had begun only since 1995 in which only a few cases with IAVFs after LDSs were included in the paper by Papadoulas (1).
Data extraction
For each included study, the following clinical data were collected: first author, year of publication, gender and age of the patient, level of disc space involved, time interval, clinical presentations, diagnostic tool, pathology, site of the AVF, method of repair, device chosen, IIA embolization, results after the repair, complications after the repair, and period of follow-up.
Statistical analysis
Because not all of the clinical data mentioned above might be collected from each publication included in this study, percentage representation (%) was calculated from all the collected clinical data.
Results
Publication description
Thirty-one publications were ultimately (4-20) included in this study (21-34). Twenty-nine were published in English while 2 were published in Chinese (9,20). There were 44 patients enrolled; 26 (59.1%) were men and 17 (38.6%) were women. However, Bingol et al. did not report the patient’s gender in their study (6). The mean age of the patients were 42 years old, with a range of 17 to 75 years old.
Level of disc space involved
The levels of disc space involved are as following: involvement of only L3–L4 disc space occurred in 1 patient (2.3%), L4–L5 in 18 patients (40.9%), and L5–S1 in 4 patients (9.1%), while both L4–L5 and L5–S1 disc spaces were involved in 6 patients (13.6%). However, the remaining 15 patients’ disc space involvements were not reported (4,5,10,17,22,25,33).
Time interval
The time interval between the LDS and recognition of the IAVF in the patients are as follows: less than 24 hours in 4 patients (9.1%), from 24 hours to 1 week in 3 patients (6.8%), from 1 week to 1 month in 9 patients (20.5%), from 1 month to 1 year in 15 patients (34.1%), and more than 1 year for 13 patients (29.5%), while the longest time interval among all the patients was 17 years (2.3%) (15).
Clinical presentation
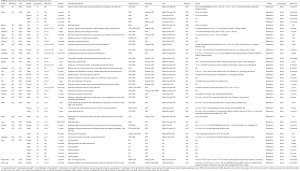
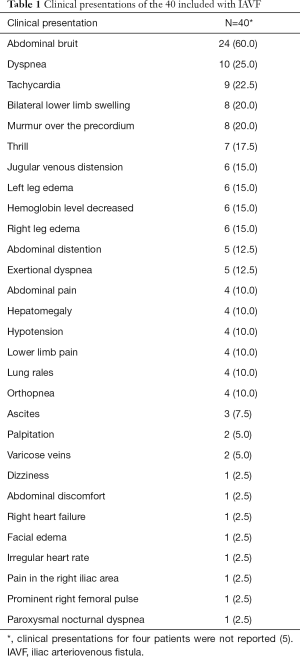
The common clinical findings were abdominal bruits (24 patients, 60.0%), dyspnea (10 patients, 25.0%), tachycardia (9 patients, 22.5%), edema of the bilateral lower extremities (8 patients, 20.0%), cardiac murmur (8 patients, 20.0%), abdominal thrill (7 patients, 17.5%), jugular venous distension (6 patients, 15.0%), left leg edema (6 patients, 15.0%), and decreased hemoglobin level (6 patients, 15.0%). Characteristics and clinical presentations of the included 44 patients are summarized in Table S1 and Table 1 respectively.
Full table
Full table
Diagnostic tool
Diagnostic tools used for the diagnosis of iliac AVFs complicating LDSs include color duplex ultrasonography (CDUS), CTA, magnetic resonance angiography (MRA), and DSA examinations. Among the diagnostic tools listed above, both CTA and DSA were used in 22 patients (50.0%); DSA was used in only 8 patients (18.2%); CTA, MRA, and DSA were used in 2 patients (4.5%); both MRA and DSA were used in 1 patient (2.3%); CDUS, CTA, and DSA were used in 2 patients (4.5%); both CDUS and CTA were used in 1 patient (2.3%); both CDUS and DSA were used in 1 patient (2.3%); and CTA alone was used in 8 patients (2.3%). However, diagnostic tools for 6 patients were not reported by their authors (13.6%) (33).
Pathology
Twenty-six patients were diagnosed as iliac AVFs only (59.1%), 16 as iliac AVFs with pseudo aneurysms (36.4%), 1 patient as iliac AVF with pseudo aneurysm and thrombus formation (2.3%), and 1 patient as iliac AVF with pseudoaneurysm and traumatic dissection of the left CIA (2.3%).
Sites of the AVFs
Vessels involved included the CIA, CIV, IVC, IIA, and IIV. Among the arteries and veins listed above, right CIA-right CIV AVFs occurred in 19 patients (43.2%), right CIA-left CIV AVFs occurred in 7 patients (15.9%), left CIA-left CIV AVFs occurred in 7 patients (15.9%), right IIA-right IIV AVFs occurred in 2 patients (4.5%), right CIA-IVC AVFs occurred in 2 patients (4.5%), and left CIA-IVC AVF occurred in only 1 patient (2.3%). However, the sites of AVF in 6 patients were not reported by their authors (13.6%) (33).
Methods and devices
Four patients with AVFs were treated with traditional open surgical repair method (9.3%) (5,6,28) while 39 patients underwent endovascular repair (90.7%). However, Mihmanli et al. (10) did not report the method of repair for the patients in their study. Sixty-two percent of the authors (n=24) reported the specific devices used for repair of AVF that varied from manufacturer to manufacturer. Details of the methods and devices are listed in Table S1.
Results and follow-ups
During the period of the follow-ups, most of the patients (97.7%) had benefited from the endovascular repair method achieving good resolutions or significant improvements without any significant complications. As for the duration of follow-up, the shortest was 3 weeks (n=1) (7), and the longest was 15 years (n=1) (33). Most of the patients were followed up for months to years (n=36).
Discussion
Prevalence
Iatrogenic vascular injury complications (including laceration, AVF, and pseudo aneurysm) after LDS may potentially be source of medical dispute between doctors and patients. This makes it difficult for it to be comprehensively reported in the literature. Furthermore, some patients suffering from vascular complications might not have any symptoms or symptoms may be present after a long time. As a result, the prevalence of iatrogenic vascular injury complications (including laceration, AVF, and pseudo aneurysm) after LDS is not clear. Nevertheless, Anda et al. (35) reported that it might vary between 1 and 5 per 10,000 LDSs. However, after retrospectively analyzing the clinical data in their institution, during the period from 1990 to 2001, Papadoulas et al. (1) stated that the prevalence of an iatrogenic vascular injury complicating with an LDS was 4 per 10,000. The prevalence of iatrogenic AVF of the iliac artery after an LDS is not clear either. In the same study by Papadoulas et al. (1), after reviewing the publications retrieved from the MEDLINE database, during the period from 1965 to 2001, they only found 66 cases reported.
This present study reports a case of iatrogenic AVF of the iliac artery after an LDS from China, which can be added to the world literature. Also, the authors of this study performed a comprehensive literature retrieval in the databases (e.g., PUBMED, EMBASE, OVID, et al.), and found a few published cases of iatrogenic AVFs of the iliac arteries complicating with LDSs. However, in contrast to the cases published in the databases mentioned above, the patient concerned in this present study suffered from AVF, pseudo aneurysm, and traumatic dissection of the left CIA at the same time, which to the author’s knowledge, is much more complex than the other published cases. As a result, it was not reported until now.
Risk factors
An iatrogenic vascular injury complication after an LDS is rare, but when it does occur, it can lead to life-threatening results. Therefore, it is important to identify the potential risk factors carefully before an LDS. The main risk factors are summarized as follows: (I) previous history of abdominal or lumbar intervertebral disc surgery which can cause adhesions between the lumbar vertebral bodies and the retroperitoneal vessels (36); (II) chronic disc diseases, for example, degeneration or disruption of the peridiscal fibrosis, anterior longitudinal ligament, or anterior annulus fibrosus (37); (III) inappropriate operation; especially inappropriate deep intrusion of the pituitary rongeur (38); (IV) inappropriate posture of patients (39); (V) pillows placed under the abdomen in the prone position while performing the procedure, which may decrease the distance between the operated disc and the retroperitoneal vessels lying in front of the disc (40); and (VI) anomalies of a lumbar vertebral body, such as a hypertrophic spur which may compress retroperitoneal vessels during the procedure (41). The patient reported from our hospital seems to not have any potential risk factors as listed above.
Clinical presentations and its pathogenesis
Anatomy plays an important role in the occurrence of IAVF after an LDS. The AA and IVC usually course along the left and right side of the spine respectively. At the middle level of the 4th lumbar vertebra, or occasionally at its inferior border, the AA branches off to the left and right CIA, which continues off the left and right internal and external iliac arteries. The common iliac veins at that level combine to form the IVC. Therefore, there is a wide vascular bed in front of the lumbar spine that is separated from each other by the anterior longitudinal ligament only. As a result, IAVFs may occur as complications of LDSs when the anterior longitudinal ligaments and the adjacent arteries and veins are perforated by instruments of the disc space, usually by a pituitary rongeur and a screw.
Vascular injury complications during LDSs are usually thought to be related with the level of lumbar intervertebral disc space. Generally, injury of the AA and IVC occur at the level of the L3–L4 intervertebral disc spaces, while the iliac arteries and veins may occur at the level of the L4–L5 and L5–S1 intervertebral disc spaces. Other vessels injured at this level, such as the internal iliac vessels (16,27), superior mesenteric artery (42), inferior mesenteric artery (32,42), superior rectal artery (43), median sacral artery (44), and L4 lumbar artery (32,42), have also been reported. The vessels affected the most during LDSs appeared to be the right common iliac arteries (1,42).
In the 44 patients with IAVFs, the top 3 most common IAVFs were right CIA-right CIV (43.2%), right CIA-left CIV (15.9%), and left CIA-left CIV (15.9%) AVFs. The patient in our study was operated at the level of the L4–L5 intervertebral disc space, causing injury to the left CIA-CIV and leading to the formation of an AVF between them along with a pseudoaneurysm and dissection of the left CIA.
When AVFs are formed between the iliac arteries and adjacent veins, abnormal channels of left to right shunts are built locally. Blood flow occurs from high-pressure vessels, e.g., iliac arteries, to vessels with low-pressure, e.g., other veins, resulting in increased blood volume in the IVC, right atrium, and ventricle. Long-term volume overload in the right heart may result in pulmonary hypertension and subsequently right ventricular hypertrophy, right heart failure, and systemic venous congestion. After that, symptoms and signs of high output heart failure, for example, enlargement of the jugular veins, hepatomegaly, enlargement of the visceral veins (e.g., superior and IVC, hepatic, pelvic floor veins, etc.), ascites, bilateral lower extremities edema, dyspnea on exertion, and cardiac murmur can occur. Also, some patients may present with abdominal masses with or without thrill on palpation, and continuous vascular bruits over the abdomen on auscultation (1,2).
The main clinical manifestations of this series of patients were bruits over the abdomen (60.0%), dyspnea (25.0%), tachycardia (22.5%), edema of the bilateral lower extremities (20.0%), cardiac murmur (20.0%), abdominal thrill (17.5%), distension of the jugular veins (15.0%), and edema of the left leg (15.0%).
Diagnosis and differential diagnosis
After exclusion of any heart, lung, and vascular disorders in a patient with history of an LDS and features of high output heart failure, abdominal mass with or without thrill on palpation, and continuous vascular bruit over the abdomen on auscultation, iatrogenic IAVF should be kept in mind.
Medical imaging examinations, such as CDUS, CTA, MRA, and DSA each plays an important role in the diagnosis, differential diagnosis, and follow-up of an iatrogenic IAVF. As were shown in the 44 patients included in this paper, CTA and/or DSA combined with or without other investigations (CDUS and MRA) were the first choices for IAVF. CTA can demonstrate the site, size, anatomical relationship between the artery and vein of an AVF accurately. Any secondary changes (e.g., thrombus formation) and/or presence of other types of vascular lesions (e.g., pseudo aneurysm or dissection) can also potentially be detected through CTA. Post-processing technologies of CTA examinations, for example, multiple plane reconstruction (MPR), curve plane reconstruction (CPR), maximum intensity projection (MIP), or volume rendering (VR), are also added benefits of CTA (45). As a result, more information and details can be provided by CTA examinations, which is why clinicians can utilize CTA to plan surgeries and evaluate the potential risks. On the other hand, although being an invasive procedure, DSA examinations are still considered a gold standard for the diagnosis of AVFs, while importantly rendering treatment at the same time. However, comparative advantage MRA possesses over CTA and DSA are that it is non-ionizing radiation, non-invasive, and has low risks of contrast-related complications in evaluating patients with AVFs (46-48). Thus, MRA is particularly helpful to patients with chronic renal disease, young or pregnant subjects, and those who require frequent examinations (without contra-indications and non-compatible stent-grafts) (46-48).
When it comes to the differential diagnosis, deep vein thrombosis, pulmonary embolism, non-high output heart failure, primary pulmonary hypertension, and some other common diseases that may originate from the heart, lung, and vascular regions, should be taken into consideration (1,2,49). Overall, a careful inquiry of the medical history, comprehensively performed physical examination, and the reasonably selected above-mentioned imaging modalities, are helpful in the formulation of diagnosis and ruling out other differential diagnoses.
The patient in this present study was finally confirmed as an iatrogenic IAVF after an LDS which was based on the following facts: (I) clinical manifestations of high output heart failure; (II) clear medical history of LDS; (III) CTA and DSA examinations confirming the diagnosis and ruling out other differential diagnoses; (IV) the patient doing well after the endovascular stent-graft placement surgery, even during the two-year period of follow-up; and (V) a few case reports in the literature with conditions similar to the patient in this study.
Treatment
Treatment choice may mainly focus on the traditional open surgical repair and relatively new endovascular repair technologies, which may depend on the type of the injury and some other clinical situations (1,2,49,50). Traditional open surgical repairs may include excision with end-to-end anastomosis, interposition grafting, suturing from within the vessel, endovascular embolization, ligation, patch angioplasty, and primary suturing (1,2,49,50).
Generally, traditional open surgical repairs may have the disadvantages of greater trauma, increased bleeding, prolonged operation duration, a much higher incidence of complications and mortality when compared with endovascular repairs (1,2,49,50). Thus, only a small number of patients with IAVFs underwent traditional open surgical repairs (1,2,49,50). The patient in our study did not experience any procedure related morbidity or mortality; however, reported operative mortality rates range from 5% to 10% (5). On the contrary, there are more surgeons and patients willing to select endovascular repairs as the treatment method. Since it was first introduced to treat a case of a common iliac artery-to-inferior vena cava fistula successfully by Zajko et al. in 1995 (51), endovascular repair has been widely used in the treatment of an IAVF after an LDS (1,2,49,50).
There were 4 patients who were treated with traditional open surgeries (9.3%) (5,6,28), and thirty-nine patients who were treated with endovascular repair methods (90.7%). IIA embolization may also be needed to prevent the circulation from contralateral IIA or other collateral pathways in the case of involved internal iliac artery (type II endoleak) (47,52-54). However, there were only three patients who were treated with IIA embolization (right IIA) in combination with endovascular repair for AVFs (7.7%, 3/39) (4,18,31). Hence, broadly speaking, the endovascular methods that may be performed during the repair may include placement of a covered-stent graft at the arterial or venous site, embolization of the affected vessel, a or combination of these approaches (7,55). As for the outcomes, although duration of the follow-ups between the publications were not consistent with each other, most patients had achieved resolution or significant improvements, and there were no significant complications that were reported which indicated that endovascular repair technology can be an ideal treatment of choice for selected patients with IAVFs after LDSs.
Iatrogenic IAVF vs. AVF occurrence in other positions
Except for the iatrogenic IAVFs associated with LDS, there are some other iatrogenic AVFs, which may be encountered when medical activities are performed. The medical activities may include transradial and transfemoral artery coronary angiography surgeries (radial and femoral AVFs), total ankle and knee arthroplasty surgeries (ankle and knee AVFs), left atrial appendage surgeries (femoral AVFs), kidney surgeries (renal AVFs), pacemaker implantation surgeries (axillary AVFs), breast core-biopsies (axillary AVFs), coronary artery bypass grafting surgeries (aortocoronary AVFs), and others (Table S2) (56-66), even though they have different clinical backgrounds. This present study reports a case of iatrogenic AVF of the iliac artery after an LDS, indicating it is another source of iatrogenic AVF in the clinical practice. Thus, it can be added to the world literature.

Full table
Among the iatrogenic AVFs listed above (Table S2) (56-66), the radial and femoral AVFs each has a relatively higher prevalence. According to the documents, patients who underwent cardiac catheterization may suffer from femoral AVFs at a rate of 0.0–0.08% (67,68), whereas radial AVFs have a rate of 0.0–0.03% (69,70). This is why the transradial approach to percutaneous coronary angiography and coronary intervention has gained its popularity among the interventional cardiologists (71). When compared with iatrogenic IAVFs complicated with LDSs, iatrogenic radial and femoral AVFs have the following clinical characteristics: (I) iatrogenic radial and femoral AVFs locate at the body surface, which makes symptoms of pulsatile masses and murmurs on the intervention site be relatively more easy to detect than IAVFs (64); (II) diagnosis of iatrogenic radial and femoral AVFs is mostly performed with CDUS, and DSA is used for definitive diagnosis (64); and (III) as for the treatments, traditional open surgical repair technologies are mostly used for symptomatic iatrogenic radial and femoral AVFs, while conservative treatments can be used for the asymptomatic ones (72-74).
Conclusions
An iatrogenic IAVF after an LDS is a rare occurrence; however, more attention should be paid to it for the purpose of obtaining accurate diagnosis and proper treatment.
Acknowledgments
Morgan A. McClure from North Sichuan Medical College’s Affiliated Nanchong Central Hospital of China’s Sichuan Province is acknowledged for his kind help in proofreading and editing this paper.
Funding: This paper was given grants for two scientific projects by the Health and Family Planning Commission of Sichuan Province (funding numbers 17PJ184 and 140107, respectively).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Papadoulas S, Konstantinou D, Kourea HP, Kritikos N, Haftouras N, Tsolakis JA. Vascular injury complicating lumbar disc surgery. A systematic review. Eur J Vasc Endovasc Surg 2002;24:189-95. [Crossref] [PubMed]
- van Zitteren M, Fan B, Lohle PN, de Nie JC, de Waal Malefijt J, Vriens PW, Heyligers JM. A shift toward endovascular repair for vascular complications in lumbar disc surgery during the last decade. Ann Vasc Surg 2013;27:810-9. [Crossref] [PubMed]
- Götze CJ, Secknus MA, Strauss HJ, Lauer B, Ohlow MA. High-output congestive heart failure due to congenital iliac arteriovenous fistula. Herz 2006;31:793-7. [PubMed]
- Hart JP, Wallis F, Kenny B, O'Sullivan B, Burke PE, Grace PA. Endovascular exclusion of iliac artery to iliac vein fistula after lumbar disk surgery. J Vasc Surg 2003;37:1091-3. [Crossref] [PubMed]
- Kwon TW, Sung KB, Cho YP, Kim DK, Ko GY, Yoon HK, Kim GE. Large vessel injury following operation for a herniated lumbar disc. Ann Vasc Surg 2003;17:438-44. [Crossref] [PubMed]
- Bingol H, Cingoz F, Yilmaz AT, Yasar M, Tatar H. Vascular complications related to lumbar disc surgery. J Neurosurg 2004;100:249-53. [PubMed]
- Wang EA, Lee MH, Wang MC, Lee HY. Iatrogenic left iliac-caval fistula: imaging and endovascular treatment. AJR Am J Roentgenol 2004;183:1032-4. [Crossref] [PubMed]
- Gallerani M, Maida G, Boari B, Galeotti R, Rocca T, Gasbarro V. High output heart failure due to an iatrogenic arterio-venous fistula after lumbar disc surgery. Acta Neurochir (Wien) 2007;149:1243-7. [Crossref] [PubMed]
- Liang ZH, Cui JG, Yu XL, Tian HQ. Endovascular stent-graft repair of a case of arteriovenous fistula between the right common iliac artery and the right common iliac vein after lumbar discectomy surgery Chinese Journal of Interventional Imaging and Therapy 2007;4:81. (in Chinese).
- Mihmanli I, Kantarci F, Ulus SO, Bozlar U, Kadioglu A, Yildirim D. Lower extremity venous pathology mimicking deep vein thrombosis: 2 case reports. Ultraschall Med 2007;28:421-5. [Crossref] [PubMed]
- O’Brien J, Buckley O, Torreggiani W. Hemolytic anemia caused by iatrogenic arteriovenous iliac fistula and successfully treated by endovascular stent-graft placement. AJR Am J Roentgenol 2007;188:W306. [Crossref] [PubMed]
- Düz B, Kaplan M, Günay C, Ustünsöz B, Uğurel MS. Iliocaval arteriovenous fistula following lumbar disc surgery: endovascular treatment with a Stent-graft. Turk Neurosurg 2008;18:245-8. [PubMed]
- Akpinar B, Peynircioglu B, Cil B, Daglioglu E, Cekirge S. Iliac vascular complication after spinal surgery: immediate endovascular repair following CT angiographic diagnosis. Diagn Interv Radiol 2009;15:303-5. [PubMed]
- Geraghty S, Durham JD, Levy JM, Wolf PS. Endovascular repair of an arteriovenous fistula after intervertebral disc surgery: case report. J Vasc Interv Radiol 2009;20:1235-9. [Crossref] [PubMed]
- Sarmiento JM, Wisniewski PJ, Do NT, Vo TD, Aka PK, Tayyarah M, et al. Bifurcated endograft repair of ilio-iliac arteriovenous fistula secondary to lumbar diskectomy. Ann Vasc Surg 2010;24:551.e13-551.e17. [Crossref] [PubMed]
- Kiguchi M, O'Rourke HJ, Dasyam A, Makaroun MS, Chaer RA. Endovascular repair of 2 iliac pseudoaneurysms and arteriovenous fistula following spine surgery. Vasc Endovascular Surg 2010;44:126-30. [Crossref] [PubMed]
- Mulaudzi TV, Sikhosana MH. Arterio-venous fistula following a lumbar disc surgery. Indian J Orthop 2011;45:563-4. [Crossref] [PubMed]
- Kim JH, Ko GY, Kwon TW, Nam GB, Cho YP. Endovascular treatment of an iatrogenic large vessel arteriovenous fistula presenting as high output heart failure: a case report. Vasc Endovascular Surg 2012;46:495-8. [Crossref] [PubMed]
- Park HK, Choe WJ, Koh YC, Park SW. Endovascular management of great vessel injury following lumbar microdiscectomy. Korean J Spine 2013;10:264-7. [Crossref] [PubMed]
- Li Y, Fu Q, Liu A, Zheng Z, Fan W, Zhu Z, Chen L, Dai W. A case of iatrogenic ilio-iliac arteriovenous fistula initially misdiagnosed as deep venous thrombosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1217-20. (in Chinese). [PubMed]
- Uei H, Tokuhashi Y, Oshima M, Miyake Y. Vascular injury following microendoscopic lumbar discectomy treated with stent graft placement. J Neurosurg Spine 2014;20:67-70. [Crossref] [PubMed]
- Yılmaz YK, Çelikbilek M, Sarıkaya S, Okur A, Çelikbilek A, Atalay T, et al. Iliac arteriovenous fistula presenting with ascites. Turk J Gastroenterol 2014;25:210-2. [Crossref] [PubMed]
- Cape H, Balaban DY, Moloney M. Endovascular repair of arteriovenous fistula after microendoscopic discectomy and lamino-foraminotomy. Vascular 2015;23:93-8. [Crossref] [PubMed]
- Huttman D, Cyriac M, Yu W, O'Brien JR. The unusual presentation of a vascular injury after lumbar microdiscectomy: case report. J Neurosurg Spine 2016;24:381-4. [Crossref] [PubMed]
- Kubelik D, Morellato J, Jetty P, Brandys T, Hajjar G, Hill A, Nagpal S. Endovascular Repair of a Chronic AV Fistula Presenting as Post-Partum High Output Heart Failure. EJVES Short Rep 2016;31:19-22. [Crossref] [PubMed]
- Park T, Park SH, Arora A. Delayed High Output Heart Failure due to Arteriovenous Fistula Complicated with Herniated Disc Surgery. J Korean Med Sci 2016;31:2051-3. [Crossref] [PubMed]
- Rohit MK, Gupta A, Khandelwal N. Endovascular Transluminal Stent Grafting: Treatment of Choice for Post Lumbar Spine Surgery Iliac Arteriovenous Fistulae. Catheter Cardiovasc Interv 2016;88:E203-E208. [Crossref] [PubMed]
- Shah VM, Nayar R. Iatrogenic iliac arteriovenous fistula with aortic pseudoaneurysm formation post lumbar (L4-L5) laminectomy and pedicular screw fixation. Korean J Anesthesiol 2016;69:305-6. [Crossref] [PubMed]
- Jung HS, Kim DJ, Kim HS, Lee HK, Choi SJN, Chung SY. Vascular Complications Related to Posterior Lumbar Disc Surgery. Vasc Specialist Int 2017;33:160-5. [Crossref] [PubMed]
- Noland S, Espinoza CA, Dvorak JD, Rose JD, Powell CS. Endovascular Repair of Iatrogenic Iliocaval Fistula Causing High-Output Cardiac Failure after Spine Fusion. Ann Vasc Surg 2017;45:262.e1-262.e5. [Crossref] [PubMed]
- Ocal O, Peynircioglu B, Eldem G, Akpinar E, Onur MR, Kabakci G. Iliac arteriovenous fistulas after lumbar spinal surgery. Turk J Emerg Med 2017;17:109-11. [Crossref] [PubMed]
- Varghese K, Adhyapak SM. Stent Migration during Endovascular Treatment of Traumatic Arteriovenous Fistula following Lumbar Spine Surgery. Ann Vasc Surg 2017;41:281.e1-281.e5. [Crossref] [PubMed]
- Gok M, Aydin E, Guneyli S, Akay A, Cinar C, Oran I. Iatrogenic Vascular Injuries Due to Spinal Surgeries:Endovascular Perspective. Turk Neurosurg 2018;28:469-73. [PubMed]
- Kaźmierski P, Wąsiewicz M, Chrząstek J, Pająk M. Endovascular treatment of iatrogenic arteriovenous fistula of the iliac vessel. Adv Clin Exp Med 2018;27:1371-5. [Crossref] [PubMed]
- Anda S, Aakhus S, Skaanes KO, et al. Anterior perforations in lumbar discectomies. A report of four cases of vascular complications and a CT study of the prevertebral lumbar anatomy. Spine 1991;16:54-60. [Crossref] [PubMed]
- Serrano Hernando FJ, Paredero VM, Solis JV, Del Rio A, Lopez Parra JJ, Orgaz A, et al. Iliac arteriovenous fistula as a complication of lumbar disc surgery. Report of two cases and review of literature. J Cardiovasc Surg (Torino) 1986;27:180-4. [PubMed]
- Bolesta MJ. Vascular injury during lumbar diskectomy associated with peridiskal fibrosis: case report and literature review. J Spinal Disord 1995;8:224-7. [Crossref] [PubMed]
- Villano M, Cantatore G, Narciso N, Santilli F, Bifano D, Cerillo A. Vascular injury related to lumbar disk surgery. Neurochirurgia (Stuttg) 1992;35:57-9. [PubMed]
- Fruhwirth J, Koch G, Amann W, Hauser H, Flaschka G. Vascular complications of lumbar disc surgery. Acta Neurochir (Wien) 1996;138:912-6. [Crossref] [PubMed]
- Raptis S, Quigley F, Barker S. Vascular complications of elective lower lumbar disc surgery. Aust N Z J Surg 1994;64:216-9. [Crossref] [PubMed]
- Sande E, Myhre HO, Witsoe E, Lundbom J, Stolt-Nielsen A, Anda S. Vascular complications of lumbar disc surgery. Eur J Surg 1991;157:141-3. [PubMed]
- Erkut B, Unlü Y, Kaygin MA, Colak A, Erdem AF. Iatrogenic vascular injury during to lumbar disc surgery. Acta Neurochir (Wien) 2007;149:511-5; discussion 516. [Crossref] [PubMed]
- Tsai YD, Yu PC, Lee TC, Chen HS, Wang SH, Kuo YL. Superior rectal artery injury following lumbar disc surgery. Case report. J Neurosurg 2001;95:108-10. [PubMed]
- Szolar DH, Preidler KW, Steiner H, Riepl T, Flaschka G, Stiskal M, Moelleken S, Norman D. Vascular complications in lumbar disk surgery: report of four cases. Neuroradiology 1996;38:521-5. [Crossref] [PubMed]
- Yan GW, Bhetuwal A, Yang GQ, Fu QS, Hu N, Zhao LW, Chen H, Fan XP, Yan J, Zeng H, Zhou Q. Congenital absence of the right coronary artery: A case report and literature review. Medicine (Baltimore) 2018;97:e0187. [Crossref] [PubMed]
- Anzidei M, Lucatelli P, Napoli A, Jens S, Saba L, Cartocci G, Sedati P, d'Adamo A, Catalano C. CT angiography and magnetic resonance angiography findings after surgical and interventional radiology treatment of peripheral arterial obstructive disease. J Cardiovasc Comput Tomogr 2015;9:165-82. [Crossref] [PubMed]
- Partovi S, Trischman T, Rafailidis V, Ganguli S, Rengier F, Goerne H, Rajiah P, Staub D, Patel IJ, Oliveira G, Ghoshhajra B. Multimodality imaging assessment of endoleaks post-endovascular aortic repair. Br J Radiol 2018;91:20180013. [Crossref] [PubMed]
- Weir-McCall JR, Bonnici-Mallia M, Ramkumar PG, Nath AF, Houston JG. Whole-body magnetic resonance angiography. Clin Radiol 2019;74:3-12. [Crossref] [PubMed]
- Liu B, Ye K, Gao S, Liu K, Feng H, Zhou F, Tian Y. The summary of experience of abdominal vascular injury related to posterior lumbar surgery. Int Orthop 2019. Epub ahead of print. [Crossref] [PubMed]
- Sexton JA, Ricotta JJ. Endovascular approaches to arteriovenous fistula. Adv Surg 2011;45:83-100. [Crossref] [PubMed]
- Zajko AB, Little AF, Steed DL, Curtiss EI. Endovascular stent-graft repair of common iliac artery-to-inferior vena cava fistula. J Vasc Interv Radiol 1995;6:803-6. [Crossref] [PubMed]
- Lee KH, Park JH, Chung JW, Han JK, Shin SJ, Kang HS. Vascular complications in lumbar spinal surgery: percutaneous endovascular treatment. Cardiovasc Intervent Radiol 2000;23:65-9. [Crossref] [PubMed]
- Yılmaz S, Erdogan A, Luleci E. Transvenous embolization and stent placement for an internal iliac arteriovenous fistula with central iliac vein occlusion. J Vasc Interv Radiol 2004;15:399-404. [Crossref] [PubMed]
- Ferro C, Petrocelli F, Rossi UG, Bovio G, Dahmane M, Seitun S. Vascular percutaneous transcatheter embolization with a new device:Amplatzer vascular plug. Radiol Med 2007;112:239-51. [Crossref] [PubMed]
- Cronin P, McPherson SJ, Meaney JF, Mavor A. Venous covered stent: successful occlusion of a symptomatic internal iliac arteriovenous fistula. Cardiovasc Intervent Radiol 2002;25:323-5. [Crossref] [PubMed]
- Moorthy N, Ananthakrishna R. Iatrogenic radial arteriovenous fistula. Heart Asia 2017;9:e010963. [Crossref] [PubMed]
- Wooster BM, Grimm NL, DeOrio JK, Mithani SK. Iatrogenic Arteriovenous Fistula With Associated Pseudoaneurysm of Posterior Tibial Artery After Revision Total Ankle Arthroplasty: A Case Report. J Foot Ankle Surg 2017;56:75-7. [Crossref] [PubMed]
- Koleilat I, Phair J. Recurrent Hemarthrosis due to Iatrogenic AVF Treated With Onyx Embolization. Vasc Endovascular Surg 2017;51:324-6. [Crossref] [PubMed]
- Rama-Merchan JC, Cruz-González I, Martín-Moreiras J, Diego-Nieto A, Rodríguez-Collado J, Sánchez PL. Percutaneous closure of iatrogenic femoral arteriovenous fistula using a covered coronary stent. Rev Port Cardiol 2017;36:219.e1-219.e4. [Crossref] [PubMed]
- Kuklik E, Pyra K, Światłowski Ł, Kuczyńska M, Sobstyl J, Drelich-Zbroja A, Jargiełło T, Tsitskari M, Szczerbo-Trojanowska M. Embolization of iatrogenic renal arteriovenous fistula-a case report. J Ultrason 2018;18:170-3. [Crossref] [PubMed]
- Gupta PK, Satheesh S, Selvaraj RJ. Percutaneous closure of iatrogenic arteriovenous fistula after pacemaker implantation. Heart Asia 2018;10:e011072. [Crossref] [PubMed]
- Barker S, Ashcroft M, Abbott N. Iatrogenic Arteriovenous Fistula of the breast following core-biopsy presenting in pregnancy. Breast J 2018;24:388-90. [Crossref] [PubMed]
- Ffrench-Constant S, Weerakoon N, Amin R, Dixon L, Taube D, Hamady M. Dual-balloon assisted super-selective embolization of high flow arterial venous fistula within a transplant kidney. CVIR Endovasc 2018;1:21. [Crossref] [PubMed]
- Işık M, Tanyeli Ö, Dereli Y, Taban VB, Altınbaş Ö, Görmüş N. Gradual Treatment of Arteriovenous Fistula in Femoral Vessels as a Complication of Coronary Angiography. Braz J Cardiovasc Surg 2018;33:631-3. [Crossref] [PubMed]
- Akbal ÖY, Hakgör A, Yılmaz F, Tanyeri S, Kaymaz C. Percutaneous treatment of high-output right heart failure with pulmonary hypertension due to a large iliac arteriovenous fistula using a patent ductus arteriosus occluder. Turk Kardiyol Dern Ars 2018;46:301-5. [PubMed]
- Saleh Y, Almaghraby A, Hammad B, Abdelkarim O, Abdelnaby M, Herzallah K, Elzawawy T. Iatrogenic aortocoronary arteriovenous fistula. Neth Heart J 2019;27:110-1. [Crossref] [PubMed]
- Kelm M, Perings SM, Jax T, Lauer T, Schoebel FC, Heintzen MP, Perings C, Strauer BE. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: implications for risk stratification and treatment. J Am Coll Cardiol 2002;40:291-7. [Crossref] [PubMed]
- Bitargil M, Başbuğ HS, Göçer H, Günerhan Y, Karakurt A. Coronary angiography our approaches to vascular complications. Turkish J Vasc Surg 2014;23:164-8.
- Eichhöfer J, Horlick E, Ivanov J, Seidelin PH, Ross JR, Ing D, Daly P, Mackie K, Ridley B, Schwartz L, Barolet A, Dzavík V. Decreased complication rates using the transradial compared to the transfemoral approach in percutaneous coronary intervention in the era of routine stenting and glycoprotein platelet IIb/IIIa inhibitor use: a large single-center experience. Am Heart J 2008;156:864-70. [Crossref] [PubMed]
- Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. RIVAL trial group. Radial Versus Femoral Access for Coronary Angiography and Intervention in Patients With Acute Coronary Syndromes (RIVAL): a randomized, parallel group, multicentre trial. Lancet 2011;377:1409-20. [Crossref] [PubMed]
- Yang JH, Gwon HC, Park JE, Song YB. Arteriovenous fistula of the wrist after transradial coronary intervention. Heart Lung 2012;41:203-6. [Crossref] [PubMed]
- Hashimoto S, Shiraishi J, Kimura M, Nishikawa M, Yanagiuchi T, Ito D, Kishita E, Yokoi H, Hyogo M, Shima T, Sawada T, Kohno Y. Usefulness of continuous compression using TR Band™ for radial arteriovenous fistula following trans-radial intervention. J Cardiol Cases 2015;12:192-4. [Crossref] [PubMed]
- Loffroy R, Chevallier O, Gehin S, Midulla M, Berthod PE, Galland C, Briche P, Duperron C, Majbri N, Mousson C, Falvo N. Endovascular management of arterial injuries after blunt or iatrogenic renal trauma. Quant Imaging Med Surg 2017;7:434-42. [Crossref] [PubMed]
- Kurzawski J, Sadowski M, Janion M. Clot injection for treatment of iatrogenic femoral arteriovenous fistula after percutaneous coronary intervention: a novel minimally invasive method. Postepy Kardiol Interwencyjnej 2016;12:364-7. [Crossref] [PubMed]