Quantification of plaque characteristics detected by dual source computed tomography angiography to predict myocardial ischemia as assessed by single photon emission computed tomography myocardial perfusion imaging
Introduction
Coronary computed tomography angiography (CCTA) is a non-invasive modality that allows the simultaneous visualization of coronary artery disease (CAD) (1). Recent developments in dual-source coronary computed tomography angiography (DSCTA) and post-processing algorithms have made semi-quantitative plaque characterization sufficiently reproducible for clinical purposes (2,3).
It has been recently demonstrated that certain quantitative and qualitative plaque features detected by CCTA are associated with the acute coronary syndrome (ACS) and adverse future cardiovascular events (2-6). Furthermore, DSCTA provides detailed anatomical information about the coronary artery and stenosis, and it is often used to evaluate patients with a low to intermediate pretest probability of CAD (6). However, the Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography I (ROMICAT I) trial demonstrated that the presence of stenosis >50% had limited diagnostic value for ACS as only 46% of patients who had obstructive CAD by CCTA had matching single-photon emission computed tomography (SPECT) perfusion abnormalities during stress testing (7-10). In addition to coronary artery stenosis, other parameters of the atherosclerotic plaque, including lesion length, plaque composition, and plaque location, are all significantly associated with myocardial ischemia (11,12). Despite these associations, these parameters have yet to be systematically evaluated in clinical practice. The evaluation of CAD is based mainly on coronary artery calcium scoring and visual evaluation of the stenosis. A more detailed evaluation and characterization of coronary artery plaque using DSCTA may improve diagnostic efficiency. However, the quantitative analysis of a large amount of coronary CTA data is time intensive and is subject to inter-observer variations. Recent progress in CT image post-processing software enables automatic quantitative analysis of the coronary artery stenosis, degree, and the plaque features, which in turn improves the accuracy and repeatability of the diagnosis (13,14). Prior studies have shown that basic plaque characteristics (plaque extent and composition) could increase the performance and specificity of CCTA in the detection of ischemia (15-18). Since more vulnerable plaque characteristic can be provided with the development on advance automatic plaque analysis software (19-28), we aim to unveil the relationship between various plaque features measured by advanced quantitative plaque analysis software using DSCTA and ischemia detected by stress/rest gated-myocardial perfusion imaging (MPI). Therefore, a comprehensive analysis of plaque features concerning the corresponding myocardial ischemic changes was performed, which is believed to add further information to the existing literature.
Methods
Study population
Consecutive patients undergoing DSCTA and stress/rest gated SPECT-MPI scans for the assessment of suspected CAD between 03/2013 and 03/2017 were included in the present investigation. Exclusion criteria included a history of coronary artery bypass graft surgery, stent placement, atrial fibrillation, prior myocardial infarction, as well as severe liver and kidney dysfunction.
DSCTA
All patients underwent either retrospectively ECG-gated or prospectively ECG-triggered protocols using a Somatom Definition 128-slice dual-source CT (Definition, Siemens Medical Solutions, Forchheim, Germany). Intravenous contrast (iopromide, 370 mg of iodine/mL; Ultravist 370, Schering, Berlin, Germany) was used in biphasic injection protocol. This gave a spatial resolution of 0.3×0.3×0.3 mm by injecting a test bolus of 10 mL contrast followed by a saline flush of 50 mL, both at a flow rate of 6 mL/s. Before scanning, breathing instructions were given, and the electrodes were placed precisely to ensure reliable ECG-triggering. β-blockers (25–75 mg metoprolol) were administrated orally 1 h before the examination in patients with heart rate >80 bpm. The effective current was 200 mA (ECG-dependent dose modulation technique was applied, full dose during the R-R interval of 40–70%), tube voltage was 120 kVp, reconstruction slice thickness =0.6 mm, reconstructed slice interval =0.5 mm and rotation time =280 ms. The pitch varied between 0.2 and 0.5 depending on heart rate and patient size. A non-contrast cardiac CT was first performed between the diaphragm and 2 cm below the carina. The contrast agent (60–70 mL, 6.0 mL/s) was injected into a median cubital vein followed by 50 mL of saline solution via a dual channel high power injector. Bolus tracking was performed in the descending aorta (ROI) with an attenuation threshold of 90 HU. The optimal diastolic and systolic phases were determined automatically.
Quantitative CCTA analysis
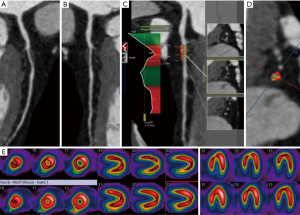
All images were reconstructed, transferred, and quantitatively analyzed using cardiovascular post-processing software (Release 5.6.5, Circle Cardiovascular Imaging; Canada). This software is capable of characterizing and quantifying 3D features, including the volume, burden, distribution, and composition of plaques. Two readers (X Yuan and T Liu), each with at least 5 years of experience in coronary CTA performed the quantitative plaque measurements in consensus. The coronary artery branches [left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) and their branches] were determined manually by the cardiovascular and the centerlines were adjusted manually when necessary. The software allows automatic, quantitative assessment of the degree of stenosis at each cross-section of the vessel. Also, the plaque constitution was quantitatively assessed at the proximal and distal ends of the maximum stenosis (parameters included volume and burden of the total, calcified, and non-calcified plaque; volume and burden of low-density non-calcified plaque (LDNCP), diameter stenosis; remodeling index, and lesion length). The severity of stenosis was quantified and divided into 4 categories: 0%= no stenosis, 1–49%= mild stenosis, 50–70%=moderate stenosis, and ≥70%= severe stenosis. The LDNCP was defined as a non-calcified plaque less than 30 HU. The burden of the plaque was defined as plaque volume multiplied by 100 per vessel volume. The maximal stenosis was assessed by calculating the ratio between minimum lumen diameter and the average value of lumen diameter in and out of the stenosis. The remodeling index is defined as the ratio of the maximum vessel diameter to a standard reference vessel diameter. The lesion length (mm) was defined as the distance between proximal-to-distal ends of the plaque at the centerline. The proximal and distal cross-sections (green lines) were determined by two radiologists with at least 5 years’ experience on cardiac CT to mark the starting and ending positions of the plaque.
Adenosine triphosphate (ATP) stress/rest gated MPI
All patients underwent an ATP stress/rest 99mTc-MIBI-gated MPI. The images were acquired on a dual-head gamma camera a [SymbiaT2, Siemens Medical, Germany, 3/8″NaI(Tl)-detector].All cameras were equipped with low-energy, high-resolution collimators. A 20% window was used with a 140-keV energy peak of technetium-99m, and data were stored in a 64×64 matrix. The patient was in the supine position. Baseline ECG was recorded before the experiments. A double venous pathway was established, one for the injection of ATP and the other for the injection of 99mTc-MIBI. Using an intravenous tracer pump, ATP was injected at 0.16 mg/(kg·min) at a constant speed for 5 min. After 3 min, 99mTc-MIBI (10 mCi) was injected from the other pathway for 20 min while the ATP was continuously administered for another 2 min. At 20 min, the patient was provided with 250 g high-fat food or 500 g whole milk and required to drink plenty of water with moderate activity to promote liver radioactivity removal. At 1-hour post-injection, the cardiac images were collected from the right anterior oblique 45° to the left posterior oblique 45° planes (5.6°/view, 25–30 sec/view, with a total of 32 frames) using a 64×64 matrix.
ATP stress/rest gated-MPI analysis
All images were analyzed and evaluated by two radiologists qualitatively with at least 5 years’ experience and certified for ECT technique in consensus (Figure 1). On a per-vessel basis, the presence of ischemia was assessed visually by radiologists in each corresponding vascular territory. Ischemia in the anterior wall and septum area was referred to the LAD supply territories. Ischemia in the lateral wall was referred to as the LCX supply territories, while the ischemia in the posterior-inferior wall indicated the localization in the RCA territories.
Statistical analysis
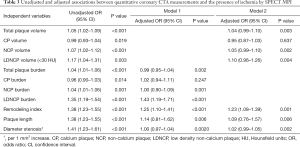
Statistical analysis was performed using commercially available statistical software (SPSS; v22.0; SPSS Inc., Chicago, IL, USA). Continuous data are expressed as a mean ± standard deviation, median, and quartile difference. Independent sample Student’s t-tests were used for between-group comparisons of continuous variables. For non-normally distributed data, a Wilcoxon signed-rank test was used. Categorical data were analyzed using a chi-square test and Fisher’s exact test. The receiver operating characteristic (ROC) curve, based on logistic regression analysis with adjusted gender and stenosis degree ≥50% as predictor variables, was used to evaluate the predictive value of plaque features for myocardial ischemia. Based on the logistic regression equation, a common predictive factor was calculated, and two models were established to predict myocardial ischemia. In the model 1, we used gender and stenosis ≥50%, and in model 2 we used gender, stenosis ≥50%, and LDNCP burden. The area under the ROC curve (AUC) was used to evaluate two models and to compare the predictive efficacy of each variable. The tests were run two-tailed, and the threshold of significance was P<0.05.
Results
Study population
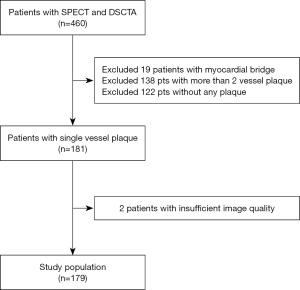
Of the 460 patients originally included in the present study, 19 patients were excluded from the final analysis due to the presence of the myocardial bridge, 122 and 138 patients were excluded due to the absence of coronary plaque and multi-vessel plaque respectively. Data for two patients were excluded due to insufficient coronary CTA image quality. Therefore, the study included a total of 179 patients (65% males) (Figure 2). The clinical characteristics and the presence of myocardial ischemia of the patients are provided in Table 1. There were more male patients in the myocardial ischemic group. However, the age and risk stratification were not significantly different between ischemic and non-ischemic groups.
Full table
Quantitative plaque assessment
A total of 533 coronary arteries in 179 patients with a single-vessel plaque were evaluated; among which, the quantitative features of plaques at the maximum diameter of stenosis were compared between the patients with and without myocardial ischemia (Table 2). Total plaque volume [25.2 (17.8–37.8) vs. 15.6 (10.3–24.9) mm3, P<0.001], calcified plaque volume (1.6±7.1 vs. 2.3±6.4 mm3, P=0.019), non-calcified plaque volume [23.6 (16.6–35.9) vs. 14.6 (10.3–22.8) mm3, P<0.001], LDNCP volume [4.9 (2.1–8.2) vs. 2.2 (1.0–5.5) mm3, P=0.003], total plaque burden (47.6%±17.1% vs. 36.2%±17.3%, P=0.002), calcified plaque burden (1.5%±5.5% vs. 2.9%±6.9%, P=0.014), non-calcified plaque burden (46.1%±18.8% vs. 33.3%±16.4%, P=0.001), LDNCP burden [12.3% (6.4–17.7) vs. 3.3% (1.6–5.3), P<0.001], remodeling index [1.2 (1.1–1.4) vs. 1.0 (1.1-1.2), P<0.001], plaque length [4.0 (3.2–6.1) vs. 3.3 (2.8–3.8) mm, P=0.009], maximum diameter stenosis [18.1% (10.0–52.9) vs. 12.9% (6.5–18.5), P=0.011] were associated with myocardial ischemia (Figure 3).
Full table
Analysis of the quantitative plaque features with predictive value for myocardial ischemia assessment
Using univariate logistic regression, LDNCP burden measured at the maximal lumen stenosis (OR 1.35; 95% CI, 1.19–1.54, P<0.001), male (OR 3.61; 95% CI, 1.55–8.42, P=0.002) and stenosis degree ≥50% (OR 14.06; 95% CI, 1.81–109.01, P=0.001) were significant predictors of myocardial ischemia, while age and cardiovascular risk factor were not.
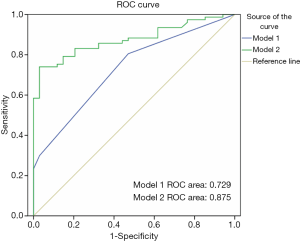
We further analyzed the predictive value of LDNCP burden. Using multivariate logistic regression analysis, LDNCP burden remained a significant predictor for myocardial ischemic after adjusting for stenosis ≥50% and gender (OR 1.33; 95% CI, 1.159–1.529, P<0.001). On the ROC curve, the AUC determined for Model 1 (using LDNCP burden, male and a stenosis ≥50%) and Model 2 (using male and a stenosis ≥50%) was 0.875 (95% CI, 0.812–0.938) and 0.729 (95% CI, 0.633–0.825), respectively (Table 3). The AUC results indicated that Model 2 was superior to Model 1 (Figure 1).
Full table
Discussion
These results suggest that quantitative plaque features (including volume and burden of total plaque, calcified plaque, NCP, and LDNCP, as well as the degree of stenosis, remodeling index, and lesion length) are associated with the incidence of myocardial ischemia in suspected CAD patients. The burden of LDNCP was a significant predictor of myocardial ischemia, independent of gender (male) and stenosis ≥50%.
This study included a patient population that underwent consecutive coronary CTA and ATP stress-rest gated MPI tests with only one vessel disease. The average stenosis of the patients is 16.6%. The results of the two tests were evaluated independently. The patient cohort was considered a low to intermediate CAD risk population. This is distinct from a previous investigation in which the relationship between coronary artery plaque and ischemia was investigated in CAD patients, especially when the invasive test was conducted (28).
It is well known that results from coronary CTA are not always indicative of myocardial ischemia (28-31). Schuijf et al. reported that of 114 patients that underwent coronary CTA and MPI Only 50% of patients with an abnormal coronary CTA (≥50% luminal narrowing) had an abnormal MPI, while 15% of patients who had no stenosis by coronary CTA showed abnormal myocardial perfusion. This inconsistency was also observed in the comparison between MPI and invasive coronary angiography (29).
Previous studies have demonstrated that atherosclerotic plaque characteristics improved the ability to discriminate ischemia by coronary CTA (30,31). Shmilovich et al. explored whether the presence of known features of vulnerable plaque on coronary CTA (low attenuation plaque content, positive remodeling, and spotty calcification) was associated with myocardial hypoperfusion in 49 patients. Their results demonstrated that the presence of low attenuation plaque and positive remodeling in severely stenotic plaques shown on a coronary CTA is strongly predictive of myocardial hypoperfusion (28). Diaz-Zamudio et al. also reported that quantitative plaque features obtained from coronary CTA are associated with ischemia by MPI independent of stenosis (32). LDNCP burden and contrast density (CD) difference are associated with ischemia in stenosis degrees of 30–69% and ≥70%, respectively. Gaur et al. conducted CTA and FFR study and found Plaque assessment and FFRCT provide improved discrimination of ischemia compared with stenosis assessment alone (31). Diaz-Zamudio et al. evaluated the relationship between multiple quantitative plaque measurements from contrary CTA and hemodynamic significance by using invasive fractional flow reserve (FFR) in 56 patients (33). Automatic quantification of the total, non-calcified, and low-attenuation non-calcified plaque burden substantially improves the determination of lesion-specific hemodynamic significance. In a large multicenter study, 254 patients underwent coronary CTA before invasive FFR measurements. Low-density NCP and FFRCT yielded significant diagnostic improvement (34). Compared with FFR, MPI provided a less direct evaluation of ischemia, especially in hypoperfused microvessels. However, MPI is noninvasive, which is preferred over FFR for the evaluation in patients at low-mid risk of CAD.
Our study further presents an insight into how different quantitative plaque features measured from CCTA alone such as LDNCP and novel markers can be used. Specifically, a larger volume and burden of plaque, more severe lumen stenosis, higher remodeling index, and longer lesion lengths indicate a high incidence of CAD. These quantitative plaque characteristics of coronary CTA significantly improved the diagnosis of myocardial ischemia. The plaque with a lipid-rich necrotic core is vulnerable to rupture and is a major cause of myocardial infarction and sudden cardiac death (34). We found that LDNCP (a coronary CTA-derived parameter equivalent to the necrotic core) is significantly associated with myocardial ischemia, which suggests that the presence of lipid-rich plaque, with its subsequent rupture, is a factor for the poor prognosis of myocardial ischemic patients. However, this conclusion requires further evaluation.
In this investigation, the gender and the extent of plaque constitutions were not explicitly investigated. However, in the prospective, multi-center Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study, a total of 697 patients (24% female) with ACSs underwent quantitative coronary angiography and radiofrequency intravascular ultrasound examinations. This study validates the notion that lesions in female have less plaque rupture and the plaque has a less necrotic core when compared with male. Other plaque features, like smaller plaque volume, may explain the similar incidence of adverse cardiac events in males. Nevertheless, it needs to be evaluated in more detail in future studies.
Given the growing body of evidence, it seems likely that quantitative analysis of plaque features has promising clinical applications for treatment planning aimed at restoring the microcirculation in the ischemic myocardium. However, plaque features are often analyzed manually, which hampers its wide application. In this study, we utilized automated methods for plaque analysis, and therefore improved its potential for clinical use. Further investigations with a greater sample size are needed to validate its clinical application and significance in therapeutic decision making.
Our study was a retrospective including 460 patients underwent both DSCTA and stress SPECT from one center and an overall referral bias could be present with patients undergoing SPECT. We excluded patients with double- and triple-vessel plaques so that the results may not be affected by balanced ischemia and imprecise definition of vascular regions in MPI. However, the normal threshold value for coronary CTA quantitative plaque analysis was not established, and coronary CTA quantitative plaque analysis is still labor intensive. Therefore, the automatic, machine learning quantitative analysis software should be developed further to improve the coronary CTA evaluation efficiency and repeatability.
Conclusions
Quantitative plaque features derived from coronary CTA are independent predictors of the myocardial ischemia detected by stress/rest gated MPI examination. To diagnose myocardial ischemia in suspected CAD patients, LDNCP burden is a significant predictor of myocardial ischemia, which is independent of significant stenosis and gender.
Acknowledgements
Funding: This study was funded by the National Nature Science Foundation of China (81871435).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135-44. [Crossref] [PubMed]
- Liu T, Maurovich-Horvat P, Mayrhofer T, Puchner SB, Lu MT, Ghemigian K, Kitslaar PH, Broersen A, Pursnani A, Hoffmann U, Ferencik M. Quantitative coronary plaque analysis predicts high-risk plaque morphology on coronary computed tomography angiography: results from the ROMICAT II trial. Int J Cardiovasc Imaging 2018;34:311-9. [Crossref] [PubMed]
- Liu T, Wang G, Li P, Dai X. Risk classification of highly sensitive troponin I predict presence of vulnerable plaque assessed by dual source coronary computed tomography angiography. Int J Cardiovasc Imaging 2017;33:1831-9. [Crossref] [PubMed]
- Ferencik M, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Liu T, Ghemigian K, Kitslaar P, Broersen A, Bamberg F, Truong QA, Schlett CL, Hoffmann U. Computed tomography-based high-risk coronary plaque score to predict acute coronary syndrome among patients with acute chest pain--Results from the ROMICAT II trial. J Cardiovasc Comput Tomogr 2015;9:538-45. [Crossref] [PubMed]
- Ferencik M, Liu T, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Pope JH, Truong QA, Udelson JE, Peacock WF, White CS, Woodard PK, Fleg JL, Nagurney JT, Januzzi JL, Hoffmann U. hs-Troponin I Followed by CT Angiography Improves Acute Coronary Syndrome Risk Stratification Accuracy and Work-Up in Acute Chest Pain Patients: Results From ROMICAT II Trial. JACC Cardiovasc Imaging 2015;8:1272-81. [Crossref] [PubMed]
- Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684-92. [Crossref] [PubMed]
- Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. [Crossref] [PubMed]
- Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724-32. [Crossref] [PubMed]
- Tsagakis K, Konorza T, Dohle DS, Kottenberg E, Buck T, Thielmann M, Erbel R, Jakob H. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg 2013;43:397-404. [Crossref] [PubMed]
- von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med 2011;154:413-20. [Crossref] [PubMed]
- Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. [Crossref] [PubMed]
- Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA 2011;306:2128-36. [Crossref] [PubMed]
- Ladapo JA, Jaffer FA, Hoffmann U, Thomson CC, Bamberg F, Dec W, Cutler DM, Weinstein MC, Gazelle GS. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol 2009;54:2409-22. [Crossref] [PubMed]
- Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, Blankstein R. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol 2013;61:880-92. [Crossref] [PubMed]
- Hacker M, Jakobs T, Hack N, Nikolaou K, Becker C, von Ziegler F, Knez A, König A, Klauss V, Reiser M, Hahn K, Tiling R. Sixty-four slice spiral CT angiography does not predict the functional relevance of coronary artery stenoses in patients with stable angina. Eur J Nucl Med Mol Imaging 2007;34:4-10. [Crossref] [PubMed]
- Naya M, Murthy VL, Blankstein R, Sitek A, Hainer J, Foster C, Gaber M, Fantony JM, Dorbala S, Di Carli MF. Quantitative relationship between the extent and morphology of coronary atherosclerotic plaque and downstream myocardial perfusion. J Am Coll Cardiol 2011;58:1807-16. [Crossref] [PubMed]
- van Velzen JE, Schuijf JD, van Werkhoven JM, Herzog BA, Pazhenkottil AP, Boersma E, de Graaf FR, Scholte AJ, Kroft LJ, de Roos A, Stokkel MP, Jukema JW, Kaufmann PA, van der Wall EE, Bax JJ. Predictive value of multislice computed tomography variables of atherosclerosis for ischemia on stress-rest single-photon emission computed tomography. Circ Cardiovasc Imaging 2010;3:718-26. [Crossref] [PubMed]
- Tamarappoo BK, Gutstein A, Cheng VY, Nakazato R, Gransar H, Dey D, Thomson LE, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Assessment of the relationship between stenosis severity and distribution of coronary artery stenoses on multislice computed tomographic angiography and myocardial ischemia detected by single photon emission computed tomography. J Nucl Cardiol 2010;17:791-802. [Crossref] [PubMed]
- Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, Dijkstra J, Delgado V, Boersma E, de Roos A, Schuijf JD, Schalij MJ, Reiber JH, Bax JJ, Jukema JW. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J 2012;33:1007-16. [Crossref] [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002;18:539-42. [PubMed]
- Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, Goddard M, Rudd JH, Bennett MR. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging 2013;6:655-64. [Crossref] [PubMed]
- Versteylen MO, Kietselaer BL, Dagnelie PC, Joosen IA, Dedic A, Raaijmakers RH, Wildberger JE, Nieman K, Crijns HJ, Niessen WJ, Daemen MJ, Hofstra L. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol 2013;61:2296-305. [Crossref] [PubMed]
- de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP, Jukema JW, Schalij MJ, Delgado V, Bax JJ, Reiber JH, Scholte AJ. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging 2013;29:1177-90. [Crossref] [PubMed]
- Oberoi S, Meinel FG, Schoepf UJ, Nance JW, De Cecco CN, Gebregziabher M, Costello P, Weininger M. Reproducibility of noncalcified coronary artery plaque burden quantification from coronary CT angiography across different image analysis platforms. AJR Am J Roentgenol 2014;202:W43-9. [Crossref] [PubMed]
- Papadopoulou SL, Garcia-Garcia HM, Rossi A, Girasis C, Dharampal AS, Kitslaar PH, Krestin GP, de Feyter PJ. Reproducibility of computed tomography angiography data analysis using semiautomated plaque quantification software: implications for the design of longitudinal studies. Int J Cardiovasc Imaging 2013;29:1095-104. [Crossref] [PubMed]
- Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr 2011;5:35-43. [Crossref] [PubMed]
- Nakazato R, Shalev A, Doh JH, Koo BK, Dey D, Berman DS, Min JK. Quantification and characterisation of coronary artery plaque volume and adverse plaque features by coronary computed tomographic angiography: a direct comparison to intravascular ultrasound. Eur Radiol 2013;23:2109-17. [Crossref] [PubMed]
- Shmilovich H, Cheng VY, Tamarappoo BK, Dey D, Nakazato R, Gransar H, Thomson LE, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 2011;219:588-95. [Crossref] [PubMed]
- Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos A, Lamb HJ, Stokkel MP, Dibbets-Schneider P, Decramer I, De Bondt P, van der Wall EE, Vanhoenacker PK, Bax JJ. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol 2006;48:2508-14. [Crossref] [PubMed]
- Park HB, Heo R. ó Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GB, Koo BK, Otake H, Budoff MJ, Berman DS, Erglis A, Chang HJ, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015;8:1-10. [Crossref] [PubMed]
- Gaur S, Øvrehus KA, Dey D, Leipsic J, Bøtker HE, Jensen JM, Narula J, Ahmadi A, Achenbach S, Ko BS, Christiansen EH, Kaltoft AK, Berman DS, Bezerra H, Lassen JF, Nørgaard BL. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 2016;37:1220-7. [Crossref] [PubMed]
- Diaz-Zamudio M, Fuchs TA, Slomka P, Otaki Y, Arsanjani R, Gransar H, Germano G, Berman DS, Kaufmann PA, Dey D. Quantitative plaque features from coronary computed tomography angiography to identify regional ischemia by myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging 2017;18:499-507. [Crossref] [PubMed]
- Diaz-Zamudio M, Dey D, Schuhbaeck A, Nakazato R, Gransar H, Slomka PJ, Narula J, Berman DS, Achenbach S, Min JK, Doh JH, Koo BK. Automated Quantitative Plaque Burden from Coronary CT Angiography Noninvasively Predicts Hemodynamic Significance by using Fractional Flow Reserve in Intermediate Coronary Lesions. Radiology 2015;276:408-15. [Crossref] [PubMed]
- Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, Malik S, Fuster V, Finn AV. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol 2013;61:1041-51. [Crossref] [PubMed]