
The risk factors associated with delirium after lumbar spine surgery in elderly patients
Introduction
Delirium is a well-defined complication in hospitalized patients characterized by an acute change in cognition with fluctuating levels of consciousness. Although delirium is often reversible, it has also been identified as a risk factor for numerous hospital complications including preventable neuropsychiatric syndrome and permanent cognitive impairment (1-8). In addition to the numerous adverse effects on an individual’s quality of life, delirium places a large burden to the healthcare system due to the prolonged hospital stay related to the extensive work-up patients undergo, and treatment once the causes have been recognized. In the postoperative setting, delirium is often not recognized despite a prevalence ranging from 11% to 51% in adult patients (4). Furthermore, a meta-analysis of more than 3,000 patients identified delirium as an independent risk factor for death, institutionalization, and dementia (9).
In concurrence with the aging population and increased life span trends, an increasing number of elderly patients are undergoing spine surgery. In particular, lumbar surgery and revision surgery are performed frequently, and the associated surgical trauma and complications have been drawing increasing attention. Delirium, for instance, a common postoperative complication, indicates adverse outcomes in patients more than 65 years old (3,4,10-13). Several studies have identified old age as a significant risk factor for postoperative delirium (1,13,14). Cognitive impairments, including delirium, are in turn correlated with complications in elderly patients (4,15). In addition, the hypoactive form of delirium is more common in elderly people and often goes unrecognized (16). Postoperative delirium following spine surgery has been reported to occur in 24.3% (17/70) of elderly patients (17). A retrospective analysis identified postoperative delirium in 13.6% (11/81) of patients following lumbar spine surgeries (18). Despite the increasing volume of spine surgeries amongst elderly patients and a high incidence of postoperative delirium, risk factors for developing delirium have not yet been systematically evaluated (19).
The origin of delirium is known to be multifactorial and may be preventable in 30–40% of all cases (4,20-22). Identifying the risk factors for developing delirium could allow for earlier intervention to prevent delirium following spine surgery, significantly reducing the associated morbidity and mortality (1,4,23,24). Currently, our understanding of the prominent risk factors is limited and further research is needed to identify preventable risk factors for postoperative delirium (4,25,26). To our knowledge, there is no prospective study that has investigated the risk factors for delirium after lumbar spine surgery. Consequently, we initiated a prospective study which aimed to explore the incidence and risk factors for postoperative delirium in elderly patients who underwent lumbar spine surgery.
Methods
Study overview
Our study enrolled 148 consecutive patients over the age of 65 years who were scheduled to undergo spine surgeries at one institution (Figure 1). Of these, 42 patients did not meet the inclusion criteria while 2 cases had to be postponed immediately prior to surgery. The remaining 104 participants (70.3%) underwent spine surgery between October 2015 and July 2016. The inclusion criteria were the following: (I) patients of age ≥65 years old, who were scheduled to undergo spinal surgery mainly consisting of discectomy, laminectomy and fusion (Figure 2); (II) hospital stay of more than 3 days; (III) indication for surgery including herniated nucleus pulposus, degenerative disc disease, spondylolisthesis, and spinal stenosis. Exclusion criteria were the following: (I) a history of delirium before surgery; (II) presence of a distinct intracranial disease such as tumor and infection; (III) patients with critical organ failure or multiple organ comorbidities known prior to surgery; (IV) patients who underwent a concurrent cervical or thoracic surgery were excluded to decrease data bias and clinical heterogeneity.
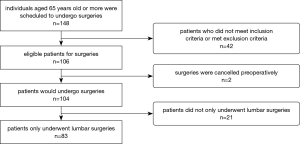
Individual characteristics and clinical data were obtained from patient interviews, caregiver statements and medical records (Table 1). Patients with postoperative delirium were assigned to a delirium group, while patients without delirium were enrolled in the control group. The investigative protocol was approved by our hospital's Institutional Review Board (NCT 02550626), and informed consent was obtained for experimentation with human subjects. This study was designed in conformity with the Declaration of Helsinki.
Full table
Assessment of delirium
Diagnosis of delirium was regularly conducted between 9 am and 12 pm on postoperative days 1–3 on the basis of the participant’s cognitive status or records from the nurse and caretaker, and observation was continued until discharge (25). Delirium was diagnosed by the previously accepted short Confusion Assessment Method (CAM) (11,27), which is a scoring system for delirium severity and has been demonstrated to have predictive validity for clinical outcomes of delirium. Participant’s cognitive status was evaluated by the Korean version of the Mini-Mental State Examination (K-MMSE) (28). The MMSE is a disease-specific tool and contains 11 items that test 5 areas of cognitive function: orientation, registration, attention and calculation, recall, and language. The maximum score is 30, with higher scores indicating better cognition. Baseline examinations were performed preoperatively in order to assess the postoperative delirium accordingly; these were performed by two independent, trained research assistants who did not participate in the surgical care of the participants in order to reduce potential subjective bias. The short CAM consists of scoring derived from the following 4 clinical assessment protocols (12,16): (I) determine the presence of an acute mental change from baseline behavior and if present, the nature of this behavior’s fluctuation course (mental status changes from hours to days); (II) determine if the patient is inattentive, easily distracted, or unable to participate in an interview; (III) evaluate the presence of disorganized thought, pressured speech or tangential speech; (IV) assess the patient’s level of consciousness (12,16). A diagnosis of postoperative delirium requires the presence of features 1 and 2 accompanied by either feature 3 or 4.
Patients were not evaluated on the day of surgery because of potential confounding due to intraoperative anesthetic medications. Patient’s characteristics, MMSE score, comorbidities, medications, preoperative laboratory findings, surgical methods, intraoperative blood loss, operation time, admission to intensive care unit (ICU), and presence of postoperative fever were examined for the purpose of identifying potential associations with postoperative delirium (Table 1).
Intraoperative and perioperative administrations
All patients underwent consistent general anesthetic regimens. Sedatives included propofol (intravenous anesthetic) and a combination of desflurane and nitrous oxide (inhaled anesthetics). Analgesic agents included remifentanil, and muscle relaxants consisted of pancuronium and vecuronium. To decrease outcome bias and clinical heterogeneity, surgical procedures and instrumentations were performed consistently for patients with the same lumbar etiology. After surgery, quantitative analgesics including fentanyl (2 µg/mL) and ropivacaine (0.15%) dosed by weight, were administered for 3 days in each patient. Antibiotics were used for 2 days in patients who underwent instrumentation. Two mg of haloperidol per 4 hours was intravenously administered for patients with postoperative delirium, and another 2 mg per hour was administered as needed for agitation.
Statistical analysis
Statistical analysis was performed with SAS version 9.4 and SPSS version 22 (IBM; Chicago, IL, USA). Numerical variables and ordinal variables were presented as mean value ± standard deviation (SD), while absolute numbers were used for categorical variables. A P value less than 0.05 was considered statistically significant. t-test was used for analyzing the data between the delirium and non-delirium (control). Chi-square test was used for the comparison of categorical data. Logistic regression analysis identified independent risk factors correlated with postoperative delirium.
Results
Eighty-three patients only underwent lumbar surgeries were finally enrolled in this study, postoperative delirium occurred in 12 patients (14.5%). Of the 12 delirium patients, 10 were diagnosed within three days after surgery, 1 was diagnosed postoperatively at day 4, and 1 was diagnosed postoperatively at day 6. The mean duration of postoperative delirium was 2.6 days (ranged from 1 to 5 days). The delirium group was composed of 8 males and 4 females with an average age of 73.4±4.9 years (ranging from 66 to 81 years). Meanwhile, the non-delirium group consisted of 19 males and 52 females with an average age of 71.0±4.6 years (P=0.102). The mean operation time was 173±67.5 minutes in the delirium group as compared to 204±85.4 minutes in the non-delirium group (P=0.229). The mean blood loss was 491±465 mL in the delirium group as compared to 684±628 mL in the non-delirium group (P=0.314). Patient information and surgical data are summarized in Table 1.
Significant differences between the delirium group and the control group
As compared to the control group, there was a higher prevalence of Parkinsonism amongst patients who developed postoperative delirium (41.7% vs. 8.5%, P=0.002). Furthermore, pre-operative C-reactive protein (CRP) was significantly higher in the delirium group as compared to the non-delirium group (7.0±15.2 vs. 1.3±2.3 mg/L, P=0.017). In addition, previous dementia, mild cognitive impairment (MCI), and prior cerebrovascular accident (CVA) or transient ischemic attack (TIA) appeared to be more common in the delirium group (both P=0.051), although these factors did not reach statistical significance (Table 1, Figure 3). Other factors including patient characteristics, MMSE score, medications, surgical methods, intraoperative blood loss, operation time, admission to ICU, and postoperative fever had no significant differences between the delirium and non-delirium groups (Table 1).
Risk factors for postoperative delirium
The results of the logistic regression analysis for identifying the risk factors for postoperative delirium are summarized in Table 2. In the univariate analysis, we found that male sex [odds ratio (OR) =0.18, 95% confidence interval (CI): 0.05–0.67, P=0.011) and Parkinsonism (OR =7.74, 95% CI: 1.87–32.0, P=0.005) were associated with delirium after lumbar surgeries. In the multivariate analysis, male sex (OR =0.10, 95% CI: 0.01–0.66, P=0.017), Parkinsonism (OR =5.83, 95% CI: 1.03–32.89, P=0.046), and lower baseline MMSE score (OR =0.71, 95% CI: 0.52–0.97, P=0.032) were independently related to postoperative delirium in the elderly patients following lumbar surgery. Additionally, admission to the ICU was observed as a factor for delirium after geriatric lumbar surgery (OR =11.18, 95% CI: 0.92–135, P=0.058), although this was not statistically significant.
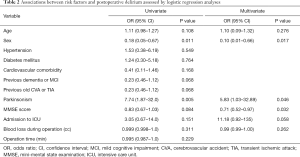
Full table
Discussion
Delirium has long been acknowledged as a significant complication in postoperative patients, with a higher prevalence amongst elderly patients; however, delirium is still frequently not recognized, evaluated, or managed appropriately (4,13). Due to the morbidity and mortality that arise from its complications, delirium needs to be identified and treated, or prevented in the perioperative setting of major surgeries including cardiothoracic surgery, organ transplantation, and spine surgery. As the frequency of spine surgery is increasing in parallel with the aging population and spinal degenerative diseases, a more comprehensive understanding of the risk factors for postoperative delirium is vital to reduce the incidence of delirium and complications following spine surgery (25). In this prospective study, 14.5% of patients over the age of 65 developed post-operative delirium. We discovered that preoperative Parkinsonism and elevated CRP levels were present more often in elderly patients who developed post-operative delirium, as compared to the control group. Additionally, male sex, Parkinsonism and lower baseline MMSE score were each independently associated with postoperative delirium in elderly patients following lumbar surgery.
Significant differences between two groups
Several statistically significant differences were identified between the delirium and non-delirium group. Patients with preoperative Parkinsonism had a higher risk for post-operative delirium (P=0.002). This finding is consistent with a retrospective study which compared 5,637 participants with Parkinsonism to 8,143 control subjects, and concluded that patients with Parkinson’s disease were treated for delirium approximately 5 times more than the control group (10.3% vs. 1.8%) (29). These differences may derive from the neurodegenerative nature of preoperative parkinsonism, thus correlating it as a risk factor for post-operative delirium (30,31). Secondly, compared with the control group, preoperative CRP was significantly higher in the delirium group (P=0.017). CRP is an acute phase protein whose level rises in response to inflammation. This inflammatory response may lead to a cascade of local neuroinflammation (32). Specifically, CRP has been considered a potential factor in neuropsychological dysfunction and may also contribute to the development of delirium by compromising the blood-brain barrier and damaging brain function (32,33). Finally, our findings noted that patients with a history of CVA/TIA and previous dementia/MCI were more common in the delirium group (Table 1). Disorders of the blood vessels may endanger oxygenation to the brain and facilitate postoperative cerebral hypoxia, which in turn contributes to the development of delirium (34).
Incidence and risk factors for postoperative delirium
Our study used short CAM and MMSE scores to diagnose delirium and measure cognitive function, respectively. This method has been validated in over 1,000 patients with a sensitivity of 94%, a specificity of 89%, and high inter-rater reliability. In this study, postoperative delirium was noted in 14.5% of patients which is a similar figure to the 19.82% of patients in Avidan et al.’s study (27). In addition to this, they found that 17.65% of patients in the 0.5 mg/kg ketamine group, and 21.30% of patients in the 1.0 mg/kg ketamine group were diagnosed with delirium after cardiac and non-cardiac surgery. Furthermore, the incidence of delirium after lumbar surgery in this study was consistent with a meta-analysis which reported post-operative delirium in 0.84% to 21.3% of patients (19).
In the present study, logistic regression analysis revealed that Parkinsonism was independently related to postoperative delirium. This finding is consistent with the Lubomski et al.’s study (29), which demonstrated that patients with Parkinsonism were more likely to suffer delirium, adverse drug reactions, and syncope as compared to their controls. A matched-pair cohort study found that Parkinsonism was a significant predictor of major postoperative complications, and delirium was the most frequently observed complication (35). The logistic regression models, including univariate and multivariate analysis, revealed that postoperative delirium occurred more frequently in male patients as compared to females. This finding is inconsistent with a meta-analysis which concluded that delirium was more common in female patients (19). Notably, MMSE score emerged as a risk factor only in multivariate regression but not in univariate regression (Table 2): a potential correlation may exist among the independent variables (risk factors such as MMSE score, age, gender, etc.) which might have led to data bias in the univariate regression model but which was eliminated in the multivariate regression model. Hence, MMSE score was an independent risk factor associated with postoperative delirium in the multivariate regression analysis of this study. In the current study, factors such as operative time and blood loss were not found to be significant intraoperative risk factors for postoperative delirium. However, a meta-analysis did note both longer operative time and severe bleeding as risk factors for postoperative delirium (19). Other intraoperative factors such as invasive or emergency procedures were also considered as risk factors for delirium following spine surgery (36). Nevertheless, there was no significant difference between surgical procedures (spinal fusion vs. compression) in the present study. Similarly, there is no consistent conclusion for the association between age and surgical outcomes of degenerative cervical myelopathy (37), although a retrospective database analysis with logistic regression demonstrated that independent predictors of delirium included age ≥65 years, depression, and psychotic disorders (38). The aforementioned inconsistencies may be related to the homogeneity of patients in this prospective study. Additionally, intraoperative procedures may decrease or increase the risk of delirium; for example, the decompression of the spinal cord carries more chance of producing confounding factors (neck immobilization, airways manipulation, failure of respiratory drive, etc.) for postsurgical delirium than decompression of the thecal sac or nerve roots (39).
Quantitative imaging was demonstrated as a predictor for cognitive decline (40,41), which may be used in predicting postoperative delirium. Wei et al. compared the regions of interest (ROIs) defined on MRI templates to reveal patients’ cognitive states between participants with early mild cognitive impairment (EMCI) and late mild cognitive impairment (LMCI) (40). They found that, between the EMCI group and the LMCI group, there were significant differences in ROIs on structural MRIs, which included imaging of the bilateral entorhinal, bilateral hippocampus, bilateral amygdala, etc. They further found that the average thickness of the left entorhinal, left middle temporal, left superior temporal, or right isthmus cingulate was the main contributor to a decreased global cognition level. Inspired by these findings, we would like to conduct another study involving the potential association between relevant ROIs on MRI templates and patient’s delirium after spine surgery.
Outlook and future investigation
Prevention or early recognition of delirium has an important role in reducing postoperative complications, and a better understanding of risk factors is necessary to effectively implement new management strategies including more precise diagnostic criteria and targeted treatments. Specifically, a clinical scoring system covering electrophysiology and neuroimaging biomarkers could be used to screen patients with varying severities of delirium and enable targeted treatments for the patients (42). These targeted treatments may include use of sedation, neuroinflammation, or neurotransmitters according to the patient’s delirium severity. To an extent, minimally invasive spine surgery may decrease post-operative delirium, as which has less invasion to normal tissues and less surgical complications (43-47). In addition, the etiology of delirium is multifactorial and will likely require a multimodal approach to be effective for its prevention and treatment (4). Defining and stratifying risk factors requires further investigation. Of note, biomarkers may have a significant role in the evaluation, diagnosis, and monitoring of delirium severity.
Limitations
There are multiple limitations to our study. Factors including pre-operative medication, anesthetics, operation segments, various laboratory findings, and postoperative fever were not stratified. The associations between stratifying risk factors and postoperative delirium may yield valuable data to guide treatment and improve outcomes. Also, only 56.1% of patients were ultimately enrolled into the study, which may have resulted in the exclusion of some risk factors that were identified by other studies. For example, preoperative depression and movement disorders were not investigated as possible risk factors for postoperative delirium although a recent study reported that these may be independent risk factors following spinal deformity surgery (48). A complete list of all factors that contribute to delirium is beyond the scope of this study as the fundamental pathophysiology of delirium has yet to be elucidated. Thus, relevant basic research needs to be conducted in order to continue pioneering the field. A multicenter randomized controlled trial with a large sample size may also help further identify and stratify the risk factors associated with postoperative delirium following lumbar surgery.
Conclusions
Post-operative delirium occurred in 14.5% of elderly patients who underwent lumbar spine surgery. Male sex, Parkinsonism, and a lower baseline MMSE score were identified as independent risk factors. These clinical findings may contribute to identifying patients at high risk for delirium to allow for prevention or earlier intervention in the post-operative course.
Acknowledgements
Funding: This study was financially supported by the grants of the China Scholarship Council (2017-3109/201708260068), faculty research grant of Yonsei University College of Medicine (6-2018-0161), and the 5511 Innovation-driven Program of Jiangxi Province Department of Science and Technology (20165BCB18017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The investigative protocol was approved by our hospital’s Institutional Review Board (NCT 02550626), and informed consent was obtained for experimentation with human subjects.
References
- Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161-74. [Crossref] [PubMed]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; 2013. Available online: http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014;370:444-54. [Crossref] [PubMed]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 2012;308:73-81. [Crossref] [PubMed]
- Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO Jr, Jones RN, Marcantonio ER, Inouye SK. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg 2015;150:1134-40. [Crossref] [PubMed]
- Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories afer postoperative delirium. N Engl J Med 2012;367:30-9. [Crossref] [PubMed]
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306-16. [Crossref] [PubMed]
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443-51. [Crossref] [PubMed]
- Flanigan PM, Jahangiri A, Weinstein D, Dayani F, Chandra A, Kanungo I, Choi S, Sankaran S, Molinaro AM, McDermott MW, Berger MS, Aghi MK. Postoperative delirium in glioblastoma patients: risk factors and prognostic implications. Neurosurgery 2018;83:1161-72. [Crossref] [PubMed]
- Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: arandomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893-902. [Crossref] [PubMed]
- Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med 2017;377:1456-66. [Crossref] [PubMed]
- Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157-65. [Crossref] [PubMed]
- Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, Donaldson MC, Whittemore AD, Sugarbaker DJ, Poss R. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994;271:134-9. [Crossref] [PubMed]
- Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993;119:474-81. [Crossref] [PubMed]
- Kim SY, Kim SW, Kim JM, Shin IS, Bae KY, Shim HJ, Bae WK, Cho SH, Chung IJ, Yoon JS. Differential associations between delirium and mortality according to delirium subtype and age: a prospective cohort study. Psychosom Med 2015;77:903-10. [Crossref] [PubMed]
- Seo JS, Park SW, Lee YS, Chung C, Kim YB. Risk factors for delirium after spine surgery in elderly patients. J Korean Neurosurg Soc 2014;56:28-33. [Crossref] [PubMed]
- Lee JK, Park YS. Delirium after spinal surgery in Korean population. Spine (Phila Pa 1976) 2010;35:1729-32. [Crossref] [PubMed]
- Shi C, Yang C, Gao R, Yuan W. Risk Factors for Delirium After Spinal Surgery: A Meta-Analysis. World Neurosurg 2015;84:1466-72. [Crossref] [PubMed]
- van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375:773-5. [Crossref] [PubMed]
- van den Boogaard M, Slooter AJC, Brüggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW, Van der Voort PHJ, Houterman S, van der Hoeven JG, Pickkers P. REDUCE Study Investigators. van der Woude, Besselink A, Hofstra LS, Spronk PE, van den Bergh W, Donker DW, Fuchs M, Karakus A, Koeman M, van Duijnhoven M, Hannink G. Effect of haloperidol on survival among critically III adults with a high risk of delirium: the reduce randomized clinical trial. JAMA 2018;319:680-90. [Crossref] [PubMed]
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516-22. [Crossref] [PubMed]
- Guo Y, Fan Y. A preoperative, nurse-led intervention program reduces acute postoperative delirium. J Neurosci Nurs 2016;48:229-35. [Crossref] [PubMed]
- Brown CH 4th, LaFlam A, Max L, Wyrobek J, Neufeld KJ, Kebaish KM, Cohen DB, Walston JD, Hogue CW, Riley LH. Delirium after spine surgery in older adults: incidence, risk factors, and outcomes. J Am Geriatr Soc 2016;64:2101-8. [Crossref] [PubMed]
- Kobayashi K, Imagama S, Ando K, Ishiguro N, Yamashita M, Eguchi Y. Risk factors for delirium after spine surgery in extremely elderly patients aged 80 years or older and review of the literature: Japan Association of Spine Surgeons with ambition multicenter study. Global Spine J 2017;7:560-6. [Crossref] [PubMed]
- Gao R, Yang ZZ, Li M, Shi ZC, Fu Q. Probable risk factors for postoperative delirium in patients undergoing spinal surgery. Eur Spine J 2008;17:1531-7. [Crossref] [PubMed]
- Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267-75. [Crossref] [PubMed]
- Shin MH, Lee YM, Park JM, Kang CJ, Lee BD, Moon E, Chung YI. A combination of the Korean version of the mini-mental state examination and Korean dementia screening questionnaire is a good screening tool for dementia in the elderly. Psychiatry Investig 2011;8:348-53. [Crossref] [PubMed]
- Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry 2015;86:324-30. [Crossref] [PubMed]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet 2009;373:2055-66. [Crossref] [PubMed]
- Amador LF, Goodwin JS. Postoperative delirium in the older patient. J Am Coll Surg 2005;200:767-73. [Crossref] [PubMed]
- Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion? Curr Opin Crit Care 2012;18:518-26. [Crossref] [PubMed]
- McGrane S, Girard TD, Thompson JL, Shintani AK, Woodworth A, Ely EW, Pandharipande PP. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care 2011;15:R78. [Crossref] [PubMed]
- Norkiene I, Ringaitiene D, Misiuriene I, Samalavicius R, Bubulis R, Baublys A, Uzdavinys G. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J 2007;41:180-5. [Crossref] [PubMed]
- Oichi T, Chikuda H, Ohya J, Ohtomo R, Morita K, Matsui H, Fushimi K, Tanaka S, Yasunaga H. Mortality and morbidity after spinal surgery in patients with Parkinson's disease: a retrospective matched-pair cohort study. Spine J 2017;17:531-7. [Crossref] [PubMed]
- Susano MJ, Scheetz SD, Grasfield RH, Cheung D, Xu X, Kang JD, Smith TR, Lu Y, Groff MW, Chi JH, Crosby G, Culley DJ. Retrospective analysis of perioperative variables associated with postoperative delirium and other adverse outcomes in older patients after spine surgery. J Neurosurg Anesthesiol 2018. Epub ahead of print. [Crossref] [PubMed]
- Tetreault L, Palubiski LM, Kryshtalskyj M, Idler RK, Martin AR, Ganau M, Wilson JR, Kotter M, Fehlings MG. Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: a systematic review of the literature. Neurosurg Clin N Am 2018;29:115-127.e35. [Crossref] [PubMed]
- Fineberg SJ, Nandyala SV, Marquez-Lara A, Oglesby M, Patel AA, Singh K. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine (Phila Pa 1976) 2013;38:1790-6. [Crossref] [PubMed]
- Kato S, Ganau M, Fehlings MG. Surgical decision-making in degenerative cervical myelopathy: anterior versus posterior approach. J Clin Neurosci 2018;58:7-12. [Crossref] [PubMed]
- Wei H, Kong M, Zhang C, Guan L, Ba M. for Alzheimer’s Disease Neuroimaging Initiative. The structural MRI markers and cognitive decline in prodromal Alzheimer’s disease: a 2-year longitudinal study. Quant Imaging Med Surg 2018;8:1004-19. [Crossref] [PubMed]
- Wu C, Guo S, Hong Y, Xiao B, Wu Y, Zhang Q. The Alzheimer’s Disease Neuroimaging Initiative. Discrimination and conversion prediction of mild cognitive impairment using convolutional neural networks. Quant Imaging Med Surg 2018;8:992-1003. [Crossref] [PubMed]
- Ganau M, Lavinio A, Prisco L. Delirium and agitation in traumatic brain injury patients: an update on pathological hypothesesand treatment options. Minerva Anestesiol 2018;84:632-40. [PubMed]
- Bae J, Lee SH. Minimally invasive spinal surgery for adult spinal deformity. Neurospine. 2018;15:18-24. [Crossref] [PubMed]
- Park CK. The successful evolution of endoscopic spine surgery: coincidence or human spirit? Neurospine 2019;16:1-2. [Crossref] [PubMed]
- Ito F, Ito Z, Shibayama M, Nakamura S, Yamada M, Yoshimatu H, Takeuchi M, Shimizu K, Miura Y. Step-by-step sublaminar approach with a newly-designed spinal endoscope for unilateral-approach bilateral decompression in spinal stenosis. Neurospine 2019;16:41-51. [Crossref] [PubMed]
- Kyoh Y. Minimally invasive endoscopic-assisted lateral lumbar interbody fusion: technical report and preliminary results. Neurospine 2019;16:72-81. [Crossref] [PubMed]
- Lee U, Kim CH, Kuo CC, Choi Y, Park SB, Yang SH, Lee CH, Kim KT, Chung CK. Does preservation of ligamentum flavum in percutaneous endoscopic lumbar interlaminar discectomy improve clinical outcomes? Neurospine 2019;16:113-9. [Crossref] [PubMed]
- De la Garza Ramos R, Goodwin CR, Jain A, Martinez-Ramirez D, Karikari IO, Sciubba DM. Inpatient morbidity after spinal deformity surgery in patients with movement disorders. J Spine Surg 2017;3:601-8. [Crossref] [PubMed]


