
Combined native magnetic resonance angiography, flow-quantifying, and perfusion-imaging for impending second-stroke assessment
Introduction
Atherosclerotic carotid artery disease is the leading cause of ischemic stroke. Spencer and Reid first postulated the hemodynamic impact of stenosis within a vessel as a curve that predicts no substantive decline in flow until approximately 70% stenosis and a steep threshold for flow restriction at approximately 80% stenosis (1). Carotid stenosis risk appears to largely correspond to these thresholds, where moderate (50–69%) stenosis has a more benign prognosis than severe stenosis, and a progressive increase in stroke risk is associated with a degree of stenosis beyond 70%. Magnetic resonance imaging (MRI) is used widely in clinical diagnoses to reveal arterial stenosis noninvasively.
MRI has emerged as a powerful means of noninvasively assessing brain pathology, and a standardized stroke MRI protocol has been employed since 1999 (2). This standard multimodal MR protocol includes diffusion-weighted imaging (DWI), T2*-weighted imaging, fluid-attenuated inversion recovery (FLAIR) imaging, T2-weighted imaging, and MR angiography (MRA). The use of a multimodal stroke MRI in the diagnostic workup of a hyper-acute stroke patient saves time and reduces costs while rendering all the critical information necessary to allow clinical doctors to initiate treatment for stroke patients. However, the current standard protocol cannot reveal the risk of stroke; it can only provide the anatomic location and stroke type. The current standard protocol can be valuable in diagnosing hyper-acute stroke, but it is not sufficient during follow-up of stroke patients or to assess the risk of the second stroke. This necessitates the introduction of other clinical techniques for prediction a second-stroke.
In this review, we introduce non-invasive MRI techniques and demonstrate the advantage of combining of MRA, phase-contrast (PC) flow imaging, and perfusion-weighted imaging for detecting the risk of a second stroke.
Noninvasive time-of-flight MRA (TOF-MRA)
For the first two decades of MRI use, TOF-MRA was the dominant non-contrast bright-blood method for imaging the morphology of the human vascular system (3).
As the name implies, it is based on the principle of flow-related enhancement, a TOF flow phenomenon. Stationary tissues in an imaged volume become magnetically saturated by multiple repetitive RF-pulses that drive down their steady-state magnetization levels. “Fresh” blood flowing into the imaged volume has not experienced these pulses and thus has a high initial magnetization. The signal from inflowing blood thus appears paradoxically bright compared to background tissue. A maximum intensity projection, which maps the projection image of voxels, show greatest signal intensity along each perpendicular projection in the plane of the desired image through the MR angiographic data set, rendering a technique with leveling, filtering, and optional surface display that is then used to create the MR angiogram.
Although now supplanted in morphological MRA by other techniques, it remains a most critical method for non-contrast neurovascular and peripheral MRA, and it has been widely used to diagnose intracranial artery vascular irregularities (4). TOF-MRA provides morphological irregularity and has enough sensitivity and specificity for detecting stenoses or occlusions, but their presence cannot directly reveal ischemia.
Cerebral blood flow (CBF) in stroke
Cerebral flow can be the primary factor in revealing ischemic stroke. Cerebrovascular risk factors such as hypercholesterolemia, hypertension, and diabetes mellitus result in atherosclerosis with a reduction of the luminal diameter and an increase in the wall-to-lumen ratio in most cerebral arteries; such changes are linked to reduced blood flow and increased ischemic damage (5). Patients with ischemic stroke can develop cerebral infarction or ischemic symptoms related to insufficient cerebral flow; therefore, flow imaging techniques can help diagnose the risk of impending stroke.
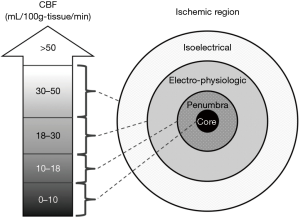
In healthy people, the ability of the cerebral vasculature to maintain CBF is relatively stable when average arterial pressures range from 60 to 150 mmHg (6,7). In acute stroke, CBF depends directly on the mean arterial pressure, which if not maintained at 60 mmHg or higher, allows the CBF to fall, leading to ischemic brain tissue. In 1948, Kety and Schmidt resolved a century-old debate (8,9), irrefutably demonstrating that CBF is regulated regionally. The electrical function of the brain is critically dependent on the CBF in the sense that a reduction beyond an ischemic threshold of approximately 15–18 mL/100-g-tissue/min (10-13) of cerebral perfusion leads to a complete failure of the somatosensory-evoked response and an ischemic penumbra (Figure S1). During such an event, the neurons remain structurally intact but functionally inactive. Those neurons can survive for some time in this state of lethargy is evidenced by the observation that a sufficient increase in CBF can restore function.
Vessel-flow quantifying by PC-MRI
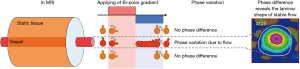
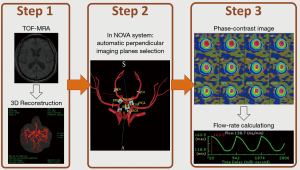
On MR magnitude images, tissues with different relaxation times can be separated by imaging contrast when scanning parameters, such as repetition time, echo time, inversion time, flip angle, and so on, are modified. In addition to magnitude imaging, the phase of an MRI can provide physiological information, such as flow rate. With the phase-induced component applied, flow information can be acquired without injecting a contrast agent for phase imaging. The dynamic tissue can show phase differences from static tissue, and the phase shift is proportional to the velocity when using a suitable velocity-encoded cine value (Figure 1) applied with one bipolar gradient. This technique is called PC-MRI. Based on vessel-flow-quantify PC-MRI, in 1997, the research team in University of Illinois at Chicago invented the Non-Invasive Optimal Vessel Analysis (NOVA, VasSol, Inc., Chicago, IL, USA) system which works with TOF-MRA to quantify CBF (14,15). Its accuracy was validated in 2007 using a canine carotid artery stenosis model (16). With automatically perpendicular imaging performed for the selected vessels, the laminar flow could be accurately measured. The laminar flow model is now used to calculate vessel flow rates (Figure 2). The vessels used to designate flow status are those that reflect the distal territory of the vertebrobasilar tree (Figure S2) (15), namely the basilar artery (BA) and the posterior cerebral arteries (PCAs).

Amin-Hanjani et al. reported that patients with the symptomatic vertebrobasilar disease who exhibit low distal flow on quantitative MRA appear to have a high risk of stroke (15). If the PCA is found to be anatomically fetal, it no longer represents a distal outflow of the vertebrobasilar system and is therefore not considered in the designation of flow status. Based on references from vessel-flow-quantifying PC-MRI, BA flow is found to be reduced if its flow is 120 mL/min, and PCA flow is found to be reduced if its flow is 40 mL/min (15).
Over one-third of ischemic strokes occur in the posterior circulation, and a leading cause is an atherosclerotic vertebrobasilar disease (17). Quantitative vessel-flow PC-MRI is a non-invasive tool to analyze the flow rate of the BA and can thus determine the risk of posterior circulation stroke. The effect of age and vascular anatomy on blood flow in major cerebral vessels has important implications for interpreting flows during the disease state (18). Using PC-MRI based NOVA tool, Amin-Hanjani et al. were able to differentiate patients with symptomatic patients of atherosclerotic vertebrobasilar disease with low posterior circulation blood flows from those with normal flows. Those patients with normal flows had a much higher stroke-free survival than those with low flows. Those with low flows was amenable to aggressive medical, interventional or surgical treatment to regain flow and then resumed a post-treatment stroke-free course that paralleled those who had normal posterior circulation blood flows (15,19). These studies represent a major advance in the diagnosis and treatment of atherosclerotic vertebrobasilar disease, as patients with flow deficits are separated into categories in which various therapies can be studied (20). Moreover, in patients with hemodynamic symptomatic vertebrobasilar disease and low flow, strict blood-pressure control (<140/90 mmHg) can increase the risk for subsequent stroke, and it has been suggested that among patients with posterior circulation occlusive disease and impaired flow, indiscriminate application of aggressive blood pressure reduction goals might not be prudent (21). It is indicated that vessel-flow-quantifying PC-MRI has the potential to serve as a powerful tool for monitoring, treating, and preventing of the second stroke.
Regional CBF-quantification based on the arterial spin-labeling technique
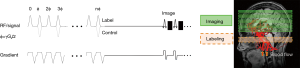
With the advantage of non-invasive acquisition, arterial spin labeling (ASL) is a technique that uses a selective inversion recovery pulse to label water within arterial blood; thus it is used as an endogenous tracer (22). Signals from distal vascular beds in the cerebral regions are acquired after a specified delay (Figure 3). These labeled images are subtracted from control images obtained without the label to generate perfusion-weighted ASL images (Figure S3). The ASL techniques commonly performed are pulsed ASL (PASL), continuous ASL (CASL), and pseudo-continuous labeling (pCASL). These techniques use a short 180° inversion pulse to invert the spins in arterial water in a slab within the neck, and the regional CBF can be acquired via image-subtraction between images with and without labeling (23).
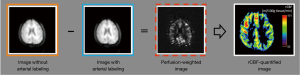
Compared to PASL, CASL provides a greater theoretical signal-to-noise ratio (SNR) and more precise control of the temporal width of the labeling blood, but it requires additional hardware and has many more magnetization transfer-related artifacts (24-27). PASL is implemented without additional hardware and uses very short radiofrequency pulses over labeling zones, but it provides relatively poor spatial coverage and a lower SNR than CASL. With the advantages of a greater SNR, better spatial coverage, few magnetization transfer artifacts, and no additional hardware required, pCASL has been used widely in clinical diagnosis (28-31); its accuracy and reliability have been reported elsewhere (32,33). The optimal label duration is determined using the relaxation time (T1) of the label and its effect on the repetition time (TR). Because clinical experience with longer labeling times is less extensive, post-labeling delays of 1,500 to 2,000 ms are currently recommended as a compromise between increasing SNR and the disadvantages of greater power deposition, T1 sensitivity, and limited clinical experience (34). In clinical use, pCASL can be used to diagnose acute and chronic ischemic stroke, which can develop insufficient cerebral perfusion (35,36). When an ischemic stroke occurs, irreversible cellular damage occurs if CBF falls below 10 mL/100g-tissue/min. Maintaining CBF at least 18 mL/100g-tissue/min or greater will maintain the potential for reversing ischemia in the tissue. When CBF decreases further, neuronal electrical silence and decreased synaptic activity can result to preserve energy stores. Therefore, pCASL can provide regional CBF and facilitate the preservation of viable neurons (5,10-15).
Combination of the TOF-MRA, PC-MRI, and 3D pCASL techniques when evaluating the risk of the second stroke
When diagnosing the risk of the second stroke, wherein the presence of an ischemic penumbra is the principal factor that determines the need for mechanical thrombectomy in those with large vessel occlusions, three procedures are recommended. First is the TOF-MRA, which can offer an initial factor to evaluate the degree of stenosis in the cerebral arteries. This factor can serve as a noninvasive risk marker for stroke. Second is flow imaging based on PC-MRI, which can be used to measure cerebral arterial flow in those vessels with stenoses; a slow flow can be a significant risk factor for stroke (11,17-19,21). A third is the use of the pCASL technique, which can help evaluate blood flow in those with stenoses in the cerebral arteries and the associated slow flow; this is used for the early diagnosis of acute ischemic stroke and chronic lacunar stroke (32). When combined with these three native sequences, MRI could be the sequential imaging solution for blood flow assessment. Herein, we present two examples that demonstrate the benefit of combining TOF-MRA, vessel-flow-quantifying PC-MRI, and ASL in the evaluation of a patient with high stroke risk. Using the values recommended elsewhere (34), two pCASL scans with post-labeling durations of 1,525 ms and 2,025 ms were used with each patient to prevent underestimating CBF.
Case 1: Arterial stenosis with low perfusion and a slow arterial flow rate
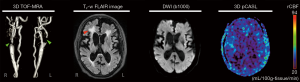
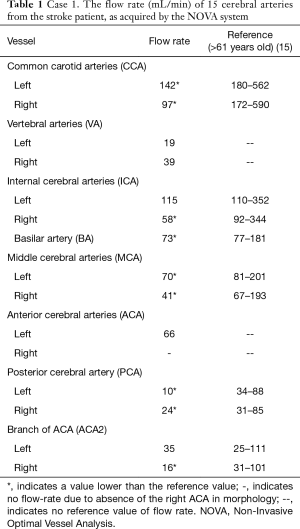
A 78-year-old woman with type 2 diabetes presented with right-sided weakness and slurred speech for 2 days. Her blood pressure was slightly elevated (135–145/63–119 mmHg), and her heart rate was standard (69–78 beats/min) before the MRI exam. TOF-MRA showed irregularity and severe stenoses in the bilateral internal carotid arteries. The DWI and FLAIR images showed bilateral periventricular lesions, suggesting subcortical arteriosclerotic encephalopathy and an acute infarct in the left coronal radiata (Figure 4). The ASL image with 2,025 ms post-labeling delay showed nearly global low perfusion (average perfusion less than 40 mL/100g-tissue/min). The results of vessel-flow-quantifying PC-MRI showed an ultra-low-flow value in nine of the 15 cerebral arteries, correlating well with ASL findings (Table 1). With the combination of TOF-MRA, PC-MRI, and pCASL, the findings indicated that this stroke patient was in an emergency condition and needed surgical intervention.
Full table
Case 2: Arterial severe stenosis with slow-flow at the BA
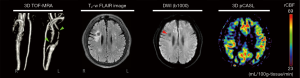
A 64-year-old man presented mild dizziness in a routine follow-up. He had a previous stroke and type 2 diabetes. His blood pressure (111–113/69–86 mmHg) and heart rate (64–75 beats/min) were normal before the MRI exam. DWI and T2-FLAIR images showed no acute infarct in the brain. TOF-MRA showed severe irregularity and stenoses in the left external carotid artery and the left proximal internal carotid artery (Figure 5). However, the ASL images with 1,525 ms of post-labeling delay showed slightly reduced perfusion in the right frontal circulation (average perfusion exceeded 40 mL/100g-tissue/min), correlating to the old infarct area. In contrast, on quantitative vessel-flow measurement, of the 15 cerebral arteries, only the BA showed slow flow (Table 2).
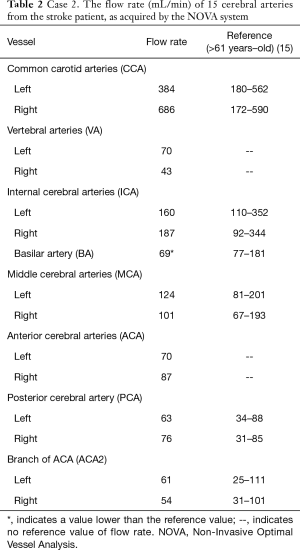
Full table
Over one-third of ischemic strokes occur in the posterior circulation, and a leading cause is an atherosclerotic vertebrobasilar disease. Reduced basilar flow has been described in patients with a vertebrobasilar disease, and those without compromised distal flow (normal blood flow values in bilateral PCAs) are at significantly lower stroke risk (17). Therefore, this patient was prescribed medication therapy only.
Limitations of the TOF-MRA, PC-MRI, and pCASL combined techniques
Some limitations should be considered when performing quantitative imaging. First, TOF-MRA can offer an evaluation of the degree of stenosis in the cerebral arteries; this factor correlates with blood flow velocity and can serve as a noninvasive risk marker for stroke. However, this technique tends to overestimate the degree and length of stenosis. Second, flow-encoded imaging is potentially an essential diagnostic technique for vascular diseases, and gradient technology combined with segmentation acquisition based on breath-measuring approaches allows imaging data to be used to eliminate respiratory artifacts. However, when using segmentation approaches, the spatial and temporal resolutions are typically compromised to accommodate the short total scanning times (37). Third, rCBF can be quantified using pCASL, but the CBF maps obtained from ASL techniques are dependent on the arterial transit time. In patients with delayed arterial transit time due to cervical or intracranial steno-occlusive arterial disease, CBF might be underestimated if the standard post-imaging delay is too short of capturing the delayed arterial spins. In that scenario, the use of multi-delay ASL might be useful to determine both the CBF and the arterial transit time accurately.
Conclusions
We reviewed the flow techniques TOF angiography, vessel-flow-quantifying PC-MRI, and pCASL and reported two cases where these imaging techniques were used with stroke patients. These three imaging techniques together can provide valuable information about arterial flow rate in the cerebral arteries necessitating surgical prevention (Case 1), or assessing the risk of posterior circulation stroke (Case 2). Also, pCASL can provide a quantitative cerebral perfusion value that reveals significant ischemia. Combining these quantitative imaging techniques provided powerful information for diagnosing an impending stroke or assessing future stroke risk, thereby facilitating appropriate clinical treatments.
Acknowledgements
Funding: The authors gratefully acknowledge partial support from the Inter-agency Research Fund from Wan Fang Hospital, Taipei Medical University, through grant No. 107-wf-swf-09.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spencer MP, Reid JM. Quantitation of carotid stenosis with continuous-wave (C-W) Doppler ultrasound. Stroke 1979;10:326-30. [Crossref] [PubMed]
- Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke 1999;30:765-8. [Crossref] [PubMed]
- Saloner D. The AAPM/RSNA physics tutorial for residents. An introduction to MR angiography. Radiographics 1995;15:453-65. [Crossref] [PubMed]
- Heiserman JE, Drayer BP, Keller PJ, Fram EK. Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology 1992;185:667-73. [Crossref] [PubMed]
- Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 2013;304:H1598-614. [Crossref] [PubMed]
- Mangat HS. Severe traumatic brain injury. Continuum (Minneap Minn) 2012;18:532-46. [Crossref] [PubMed]
- Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI, et al. Blood pressure, cerebral blood flow, and brain volumes. The SMART-MR study. J Hypertens 2010;28:1498-505. [Crossref] [PubMed]
- Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 1948;27:484-92. [Crossref] [PubMed]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948;27:476-83. [Crossref] [PubMed]
- Sundt TM, Sharbrough FW, Anderson RE, Michenfelder JD. Cerebral blood flow measurements and electroencephalograms during carotid endarterectomy. 1974. J Neurosurg 2007;107:887-97. [Crossref] [PubMed]
- Michenfelder JD, Sundt TM, Fode N, Sharbrough FW. Isoflurane when compared to enflurane and halothane decreases the frequency of cerebral ischemia during carotid endarterectomy. Anesthesiology 1987;67:336-40. [Crossref] [PubMed]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 1981;12:723-5. [Crossref] [PubMed]
- Jones MD, Traystman RJ, Simmons MA, Molteni RA. Effects of changes in arterial O2 content on cerebral blood flow in the lamb. Am J Physiol 1981;240:H209-15. [PubMed]
- Zhao M, Amin-Hanjani S, Ruland S, Curcio AP, Ostergren L, Charbel FT. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol 2007;28:1470-3. [Crossref] [PubMed]
- Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke 2005;36:1140-5. [Crossref] [PubMed]
- Calderon-Arnulphi M, Amin-Hanjani S, Alaraj A, Zhao M, Du X, Ruland S, Zhou XJ, Thulborn KR, Charbel FT. In vivo evaluation of quantitative MR angiography in a canine carotid artery stenosis model. AJNR Am J Neuroradiol 2011;32:1552-9. [Crossref] [PubMed]
- Amin-Hanjani S, Rose-Finnell L, Richardson D, Ruland S, Pandey D, Thulborn KR, Liebeskind DS, Zipfel GJ, Elkind MS, Kramer J, Silver FL, Kasner SE, Caplan LR, Derdeyn CP, Gorelick PB, Charbel FT. VERiTAS Study Group. Vertebrobasilar Flow Evaluation and Risk of Transient Ischaemic Attack and Stroke study (VERiTAS): rationale and design. Int J Stroke 2010;5:499-505. [Crossref] [PubMed]
- Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab 2015;35:312-8. [Crossref] [PubMed]
- Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, Elkind MS, Zipfel GJ, Liebeskind DS, Silver FL, Kasner SE, Aletich VA, Caplan LR, Derdeyn CP, Gorelick PB, Charbel FT. Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke Study Group. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol 2016;73:178-85. [Crossref] [PubMed]
- Ausman JI, Liebeskind DS, Gonzalez N, Saver J, Martin N, Villablanca JP, Vespa P, Duckwiler G, Jahan R, Niu T, Salamon N, Yoo B, Tateshima S, Buitrago Blanco MM, Starkman S. A review of the diagnosis and management of vertebral basilar (posterior) circulation disease. Surg Neurol Int 2018;9:106. [Crossref] [PubMed]
- Amin-Hanjani S, Turan TN, Du X, Pandey DK, Rose-Finnell L, Richardson D, Elkind MS, Zipfel GJ, Liebeskind DS, Silver FL, Kasner SE, Gorelick PB, Charbel FT, Derdeyn CP. VERiTAS Study Group. Higher stroke risk with lower blood pressure in hemodynamic vertebrobasilar disease: analysis from the VERiTAS Study. J Stroke Cerebrovasc Dis 2017;26:403-10. [Crossref] [PubMed]
- Viallon M, Cuvinciuc V, Delattre B, Merlini L, Barnaure-Nachbar I, Toso-Patel S, Becker M, Lovblad KO, Haller S. State-of-the-art MRI techniques in neuroradiology: principles, pitfalls, and clinical applications. Neuroradiology 2015;57:441-67. [Crossref] [PubMed]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed 1997;10:237-49. [Crossref] [PubMed]
- Wong AM, Yan FX, Liu HL. Comparison of three-dimensional pseudo-continuous arterial spin labeling perfusion imaging with gradient-echo and spin-echo dynamic susceptibility contrast MRI. J Magn Reson Imaging 2014;39:427-33. [Crossref] [PubMed]
- Ferré JC, Bannier E, Raoult H, Mineur G, Carsin-Nicol B, Gauvrit JY. Arterial spin labeling (ASL) perfusion: techniques and clinical use. Diagn Interv Imaging 2013;94:1211-23. [Crossref] [PubMed]
- Shin DD, Liu TT, Wong EC, Shankaranarayanan A, Jung Y. Pseudocontinuous arterial spin labeling with optimized tagging efficiency. Magn Reson Med 2012;68:1135-44. [Crossref] [PubMed]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488-97. [Crossref] [PubMed]
- Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, Hattori K, Teraishi T, Ito K, Kunugi H. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res 2014;154:113-8. [Crossref] [PubMed]
- Teune LK, Renken RJ, de Jong BM, Willemsen AT, van Osch MJ, Roerdink JB, Dierckx RA, Leenders KL. Parkinson's disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. Neuroimage Clin 2014;5:240-4. [Crossref] [PubMed]
- Boudes E, Gilbert G, Leppert IR, Tan X, Pike GB, Saint-Martin C, Wintermark P. Measurement of brain perfusion in newborns: pulsed arterial spin labeling (PASL) versus pseudo-continuous arterial spin labeling (pCASL). Neuroimage Clin 2014;6:126-33. [Crossref] [PubMed]
- Nordin LE, Li TQ, Brogren J, Johansson P, Sjögren N, Hannesdottir K, Björk C, Segerdahl M, Wang DJ, Julin P. Cortical responses to amphetamine exposure studied by pCASL MRI and pharmacokinetic/pharmacodynamic dose modeling. Neuroimage 2013;68:75-82. [Crossref] [PubMed]
- Heijtel DF, Mutsaerts HJ, Bakker E, Schober P, Stevens MF, Petersen ET, van Berckel BN, Majoie CB, Booij J, van Osch MJ, Vanbavel E, Boellaard R, Lammertsma AA, Nederveen AJ. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with 15O H2O positron emission tomography. Neuroimage 2014;92:182-92. [Crossref] [PubMed]
- Kilroy E, Apostolova L, Liu C, Yan L, Ringman J, Wang DJ. Reliability of two-dimensional and three-dimensional pseudo-continuous arterial spin labeling perfusion MRI in elderly populations: comparison with 15O-water positron emission tomography. J Magn Reson Imaging 2014;39:931-9. [Crossref] [PubMed]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-16. [Crossref] [PubMed]
- Guo L, Zhang Q, Ding L, Liu K, Ding K, Jiang C, Liu C, Li K, Cui L. Pseudo-continuous arterial spin labeling quantifies cerebral blood flow in patients with acute ischemic stroke and chronic lacunar stroke. Clin Neurol Neurosurg 2014;125:229-36. [Crossref] [PubMed]
- Grandin CB. Assessment of brain perfusion with MRI: methodology and application to acute stroke. Neuroradiology 2003;45:755-66. [Crossref] [PubMed]
- Doyle M, Kortright E, Anayiotos AS, Elmahdi AM, Walsh EG, Fuisz AR, Pohost GM. Rapid velocity-encoded cine imaging with turbo-BRISK. J Cardiovasc Magn Reson 1999;1:223-32. [Crossref] [PubMed]


