
Partial splenic embolization with Glubran®2/Lipiodol® mixture for oncological patients with hypersplenism-related thrombocytopenia requiring systemic chemotherapy
Introduction
Thrombocytopenia related to hypersplenism is seen in a variety of clinical settings, the most common being portal hypertension and splenomegaly due to cirrhosis (1,2). Thrombocytopenia of hypersplenism, defined as a platelet count below 75×109/L, is predominantly caused by increased splenic sequestration or the destruction of platelets (1). In oncological patients, hypersplenism can occur as a result of hepatic injury secondary to chemotherapy drugs, and thrombocytopenia can preclude or limit administration of systemic therapy (2-5). Platelet transfusion, which remains the most effective method of correcting thrombocytopenia, has temporary and limited effects in patients with an increase in platelet sequestration and destruction (6,7). Then, splenectomy is often considered as a last resort to treat refractory thrombocytopenia. However, surgical treatment is risky and often times contra-indicated in such poor candidates (8,9). Over the past 2 decades, partial splenic embolization (PSE) has been used to safely palliate the effects of hypersplenism by reducing the volume of splenic parenchyma (2-6). Although the efficacy of PSE for relieving thrombocytopenia is well-established, the optimal embolic agent to be used remains to determine (10-14). Various embolic materials have been used for PSE, including temporary agents such as absorbable gelatin sponge particles and permanent agents such as calibrated microspheres. All of these agents, however, are associated with a severe postembolization syndrome that often persists over 1 week. Liquid embolic agents, especially cyanoacrylates, may be very useful in such a setting, thanks to their properties (15). N-butyl cyanoacrylate-methacryloxy sulfolane (NBCA-MS; Glubran®2; GEM Srl, Viareggio, Italy) is a well-known glue comprising a proprietary comonomer, making the glue more stable. This is the only glue approved by the European Community for internal human use. This synthetically derived glue shows rapid polymerization (1–5 s), but complete sealing occurs in approximately 5 min. The use of Lipiodol® (Guerbet, Aulnay-sous-Bois, France) is mandatory to make the glue radiopaque and modulate the delay of polymerization. To our knowledge, the use of glue in such a setting has not been reported.
The purpose of the current study was to evaluate the safety, feasibility and effects on platelet count of PSE with Glubran®2/Lipiodol® mixture for oncological patients with hypersplenism-related thrombocytopenia requiring systemic chemotherapy (SC).
Methods
Study population
Retrospective single-center review of all cancer patients with chemotherapy-induced thrombocytopenia related to hypersplenism and requiring SC who underwent PSE with N-butyl cyanoacrylate-methacryloxy sulfolane (NBCA-MS) Glubran®2 glue between February 2015 and September 2017 to correct thrombocytopenia and administer SC. Patients with renal insufficiency, allergy to contrast medium or life-expectancy <3 months were excluded from the study.
The study was approved by our Institutional Review Board and a waiver was granted for informed consent.
PSE procedure
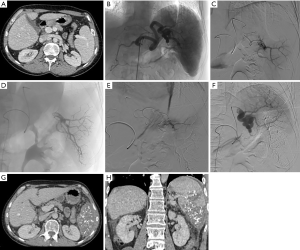
All PSE procedures were performed under intravenous sedation by 2 interventional radiologists with 6 and 12 years of experience, with the use of an Allura Xper FD 20 angio room system (Philips, Eindhoven, The Netherlands). Patients underwent diagnostic angiography under local anaesthesia by femoral approach through a 5.0-French sheath (Terumo, Tokyo, Japan). Selective cannulation of the splenic artery was then performed. Pre, per, and post angiography was carried out to identify left gastroepiploic artery, short gastric branches and pancreatic body and tail branches in order to avoid nontarget embolization. Following microcatheterization of branches supplying the splenic lower pole with a 2.7-Fr Progreat® microcatheter (Terumo, Tokyo, Japan), embolization was performed with a 1:5 ratio mixture of NBCA-MS (Glubran®2, GEM Srl, Viareggio, Italy) and Lipiodol® Ultra-Fluide (Guerbet, Aulnay-sous-Bois, France) to allow a distal embolization (Figure 1). The microcatheter was previously flushed with 5% dextrose solution. Splenic artery lower pole branches were preferentially occluded in order to minimize the occurrence of pleural effusion or subdiaphragmatic abscess. Then, injection of the mixture was slowly performed under fluoroscopy guidance to visualize the glue distribution until complete occlusion of the feeding branches (Figure 1). Postembolization 2D arteriogram and 3D Xper-CT imaging scan were used to estimate the percentage of splenic parenchyma occlusion. The embolization target was to obtain between 50% and 70% of infarcted spleen tissue, and this goal was estimated by the operator at the end of every procedure. A FemoSeal™ (Terumo) vascular closure device was systematically used at the femoral puncture site.
Regarding periprocedural medication, all patients received intravenous analgesia with 1 g of paracetamol during PSE and intravenous morphine if needed at the operator discretion. Patients were kept overnight for intravenous analgesia and antiemetic medications. They all took oral pain medication for 7 days and received empiric antibiotic coverage with amoxicilline 1 g per os/day for 10 days after PSE. Pneumococcal vaccine (Pneumovax, Merck) was systematically given before the procedure despite partial feature of splenic embolization.
Data collection
In the framework of this study, each patient’s demographic data, clinical presentation, etiology, laboratory values, and interventional data were collected. Information on length of hospital stay after the procedure, hospital readmissions, complications, and survival were obtained by review of the medical records. Duration of scopy and dose area product (DAP) were also recorded for each patient as radiation parameters during embolization procedure.
Pre- and post-procedural contrast-enhanced CT scan allowed to evaluate functional splenic volume with the post-treatment software “AW Volume Share 5-Volume Viewer” (General Electric, Milwaukee, WI) by propagation of the manual contours of the total splenic volume. Splenic width and infarcted volumes were estimated by CT scan performed between 15 and 30 days after PSE, by manual contouring of necrotic and whole part of splenic parenchyma. The splenic infarction rate (%) was then calculated by using the following formula: splenic infarction rate (%) = (infarcted splenic volume/post-PSE splenic volume) ×100.
The primary and secondary endpoints of the present study included a platelet count increase >150×109/L and the initiation of SC, respectively. The arbitrary platelet count of 150×109/L was chosen as a value that was sufficiently above the minimum thresholds commonly used in clinical practice for the safe administration of SC that impact bone marrow function (16-19). Periprocedural laboratory values were recorded. Platelet, white blood cell and red blood cell counts was monitored immediately before PSE and at days 10, 20 and 30 after PSE.
All adverse events were recorded and classified as minor or major complications according to the Society of Interventional Radiology classification system.
Statistical analysis
Values are presented as means for quantitative variables and as percentages for qualitative variables. Summary statistics were calculated for platelet count as well as white blood cell and red blood cell counts. Changes in blood cell counts over time were analyzed with a linear mixed model according to time, taking into account potential staggering variables. A P value of ≤0.05 was considered significant. Statistical analyses were performed using STATA software (version 12.0, STATA corp., College Station, Texas, USA).
Results
Patient characteristics
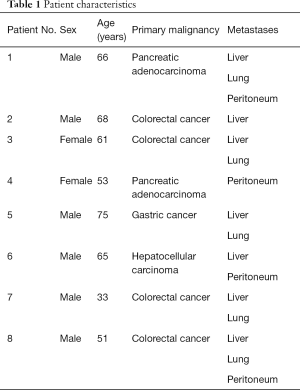
Between February 2015 and September 2017, 8 patients (6 males, 2 females; median age, 59 years; range, 33–75 years) underwent PSE for the purpose of correcting thrombocytopenia to administer optimally dosed SC. All patients had malignancies arising from the gastrointestinal tract at the time of PSE (Table 1). Primary malignancies included colorectal cancer (n=4), pancreatic adenocarcinoma (n=2), hepatocellular carcinoma (n=1), and gastric cancer (n=1). Hepatic and lung metastases were present in 7 and 5 patients, respectively. Hypersplenism was the suspected cause of thrombocytopenia in all patients based on clinical and radiological assessment. Furthermore, pre-procedural CT scan imaging revealed a mean splenic width of 156 mm (range, 126–212 mm) for the patient cohort, which is consistent with splenomegaly. Total pre-procedural splenic volume was estimated at 802 cm3 (range, 397–1,727 cm3) (Table 2).
Full table
Full table
PSE outcomes
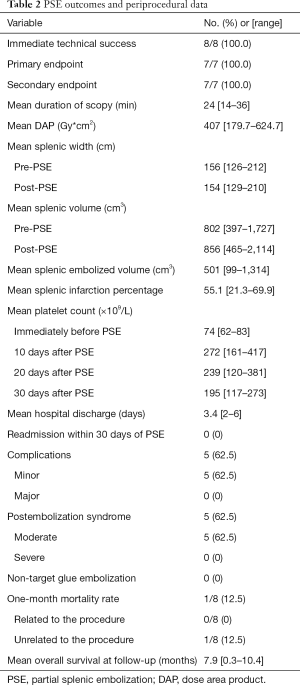
PSE was technically successful in all patients (100%). The mean duration of scopy was 24 min (range, 14–36 min). Mean DAP was 407 Gy*cm2 (range, 179.7–624.7 Gy*cm2). CT scan was performed at 19 days on average after PSE (range, 15–30 day). Mean post-PSE splenic width and volume were 154 mm (range, 129–210 mm) and 856 cm3 (range, 465–2,114 cm3), respectively. Mean post-PSE splenic infarction was 55.1% (range, 21.3–69.9%).
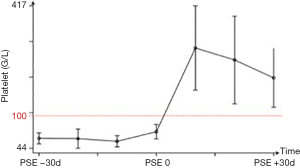
The mean platelet count immediately before PSE was 74×109/L [range, (62–83) ×109/L], confirming low platelet count for all patients prior to PSE. For procedures with available laboratory follow-up, primary endpoint was achieved in 7 (100%) of 7 patients with one-single procedure. The mean platelet count significantly increased after PSE (P<0.05) and peaked at 272×109/L [range, (161–417) ×109/L] 10 days after PSE. Mean platelet count at days 20 and 30 post-PSE were 239×109/L [range, (120–381) ×109/L] and 195×109/L [range, (117–273) ×109/L], respectively (Figure 2).
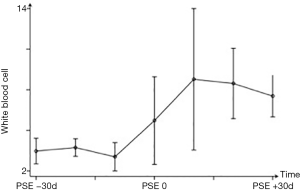
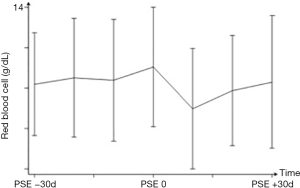
After PSE, the same trend of changes was observed for white blood cell count but with no significant peak level (Figure 3). Red blood cell count showed no significant changes over time, even after PSE (Figure 4).
The secondary endpoint of SC administration was achieved in all patients with adequate follow-up (7 of 7 patients) within 30 days after PSE. No repeat PSE was performed. Mean hospital discharge was 3.3 days after PSE (range, 2–6 days). No readmission within 30 days of PSE occurred. PSE outcomes are summarized in Table 2.
Complications
No non-target glue embolization occurred. No puncture site hematoma was noted. No major complication or grade 3 or above adverse events were reported. No severe postembolization syndrome occurred. Five (62.5%) patients experienced transient and moderate postembolization syndrome. Four patients (50%) referred low to moderate abdominal pain, 3 patients (37.5%) had low-grade fever, and 3 patients (37.5%) had nauseas. The mean overall survival was 7.9 months (range, 0.3–10.4 months) among the 8 patients after PSE. One patient with pancreatic adenocarcinoma died within 10 days of PSE because of disease progression and had no biological/radiological follow-up (Table 2).
Discussion
All patients of the present study had documented splenomegaly based on imaging criteria and had hypersplenism-induced thrombocytopenia secondary to chemotherapy-associated hepatic injury. The primary and secondary endpoints included a platelet count increase >150×109/L and the initiation of SC, respectively. For procedures with available laboratory follow-up, primary endpoint was achieved in 7 (100%) of 7 patients with one-single procedure. The mean platelet count significantly increased after PSE (P<0.05) and peaked at 272×109/L [range, (161–417) ×109/L] 10 days after PSE. The secondary endpoint of SC administration was achieved in all patients with adequate follow-up (7 of 7 patients) within 30 days after PSE.
PSE has become a good and efficient alternative to splenectomy for the treatment of refractory hypersplenism-related thrombocytopenia in oncologic patients requiring SC (2-7). Such patients are often late stage and poor candidates for splenectomy, requiring palliative treatment (8,9). Successful management of their thrombocytopenia is essential to give them access to further chemotherapy. PSE is considered relatively safe (16-18). Postembolization syndrome (PES), consisting of abdominal pain, low-grade fever and nausea is a common expected side effect of embolization of a solid organ and was encountered moderately in 62.5% of patients in the present study. No severe PES was noted here. However, small numbers of major complications including splenic abscess or rupture, reactional pleural effusion and pneumonia have been reported. None of the patients in the current series developed splenic abscess. The rate of severe complications varies in relation to the volume of splenic infarction. If it is more than 70% of the splenic volume, severe complications develop in 50% compared to 8.8% if the infarctus is between 50% and 70%. Thus, it is admitted that infarctus should be between 50% and 70%, to ensure effective management of hypersplenism and reduce severe complications (4,5,16-18) In our study, most of the patients had a 50–70% infarction area with no major complications. In 100% of our cases, patients were kept overnight for symptomatic treatment. This not consistent with more recent publications in which PSE was often times conducted as an outpatient procedure (19). However, even if the risk-benefit ratio supports PSE in these poor patients who require palliative treatment, the risk of severe complications makes preferable keeping them overnight if symptomatic treatment is needed. On the same way, although no recommendation exists regarding specific precautions against infection with PSE such as pneumococcal vaccination or prophylactic antibiotics, all patients in the present study were treated with these two options to minimize the risk of infectious complications, as those we can see after splenectomy, by analogy
SC treatments, which involve oxaliplatin, have been linked to the appearance of distinctive liver lesions, such as hepatic sinusoids blockade, sclerosis of the perisinusoidal space, and veno-occlusive disease, affecting the normal hepatic parenchyma and occasioning portal hypertension (5,19-23). Increases in spleen size, platelets splenic sequestration, and development of thrombocytopenia are surrogates of this toxicity. In our study, drug toxicity was identified as the main indirect cause of thrombocytopenia in all patients.
The arbitrary platelet count of 150×109/L was chosen as a value that was sufficiently above the minimum thresholds commonly used in clinical practice for the safe administration of SC that impact bone marrow function. A successful platelet increase above this threshold value was achieved in 100% of patients (7). In addition, the mean peak platelet value of 272×109/L achieved in the current study is comparable to that achieved in most of other reported series (7,11,19,20). Subsequently, SC was successfully administered after the procedure in all patients with adequate follow-up.
The choice of the best embolic agent is still controversial. To our knowledge, there is no series in the literature reporting results of PSE in the treatment of hypersplenism-related thrombocytopenia with the use of NBCA-MS Glubran®2 glue. As far as we are aware, this is the first report reporting the use of glue. Uncharged microparticles such as microspheres are the most commonly used for embolization in previous reports but the risk of ischemic complication may be higher than that with proximal agents such as coils or plugs (12-14). Furthermore, it might be difficult to determine the appropriate size of particles to be used based on angiography. Mechanical agents result in occlusion of the splenic artery but with minimal change in the splenic parenchyma unless the collateral network is affected. The mechanism of action of coils and plugs is entirely occlusive and their effect is equivalent to surgical ligation of the main splenic artery. One disadvantage of these mechanical embolic agents is that they are not as effective in the presence of collateral vessels, which may lead to recanalization. The main goal of PSE is to superselectively destroy part of the spleen. The intra-parenchymatous branches of the splenic artery must, therefore, be occluded as distally as possible. Embolic agents causing distal embolization such as microparticles or resorbable gelatin may therefore be used (13,14). According to Zhu et al., microparticles are more effective than absorbable gelatin in increasing the platelet count, with no difference in the rate of complications. However, the procedure can be time-consuming with microparticles. NBCA-MS (Glubran®2) was chosen in this study for embolization. Glubran®2 is a well-known surgical glue in which NBCA is combined with another monomer, methacryloxy sulfolane (MS), to produce a more pliable and stable polymer whose milder exothermic reaction (45 °C) results in less inflammation and histotoxicity than Histoacryl® or Trufill®, which are respectively not allowed for endovascular purpose and not available in Europe (15). Cyanoacrylate glues in general, and NBCA-MS Glubran®2 in particular, have the advantage of offering permanent embolization, independently of coagulation disorders. Another advantage is that the use of glue for PSE is fast and may lead to shorter duration of scopy, lower DAP and less radiation. Furthermore, embolization with NBCA-MS Glubran®2 is cost-effective in comparison with other embolic agents (24). One cc, which is the standard dose used for each patient in our series, is 3 times cheaper than any detachable coil, plug or vial of microparticles. One technical aspect is that cyanoacrylate should be mixed with Lipiodol to make the mixture radiopaque, and released under fluoroscopic control. The mixture with Lipiodol also modulates the polymerization rate. In all of our patients, we used a glue-Lipiodol ratio of 1:5, thus achieving distal intraparenchymal embolization. Indeed, the goal here is to obtain a distal embolization. For this purpose, it is needed to use a higher ratio than usual (1:3) in order to delay the polymerization of the glue in contact with blood and allow the mixture reach the distal parenchymal vascular bed before polymerization (15). Cyanoacrylate is more viscous than microparticles (24-27). The possibility of significant ischemic complications is therefore unlikely. The last advantage of the glue/lipiodol mixture is the high radiopacity. It allows to accurately check the distribution of the embolic agent into the splenic parenchyma during the angiography. It might be very useful in achieving a more reliable analysis of the embolized spleen, especially on per-procedural cone-beam CT imaging or during CT scan follow-up, even without injection of contrast medium (6,15,24). Glue embolic agents however present some drawbacks. Possible complications of cyanoacrylate PSE are glue migration in non-target vessels and a glued catheter (15,25,26). We had no such complications in our series because the glue embolization was performed by radiologists with considerable experience in the use of tissue adhesives.
Our study has some limitations. First, this is a single-center study with a small number of patients. Second, there is no control group. Comparing PSE with the use of other embolic materials might help determine the best embolic agent. Furthermore, a control group aiming for a smaller splenic infarcted area to determine the best embolization fraction could be of interest. Third, ideal criteria and perfect timing for the selection of patients should be well established to determine eligibility for the procedure. As for last limitation, this includes short follow-up time.
In conclusion, this study demonstrates that PSE with NBCA-MS Glubran®2 glue is safe and effective in the management of thrombocytopenia related to hypersplenism in cancer patients. It allows sufficient platelet count improvement for administration of SC and confirms that aiming for a 50–70% infarcted area may be sufficient to achieve success without any complications. This finding, combined with the lack of severe PES after PSE, makes it an excellent therapeutic option in the management of hypersplenism-related thrombocytopenia, allowing patients to receive SC with minimal procedure-related morbidity. Future larger studies are needed to confirm the safety and efficacy of this intervention with glue. If so, cyanoacrylate glue may prove to be the preferable agent for PSE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee. Informed consent was waived.
References
- Perry MC, Doll DC, Freter CE. Chemotherapy source book, 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2012.
- Spigos DG, Jonasson O, Mozes M, Capek V. Partial splenic embolization in the treatment of hypersplenism. AJR Am J Roentgenol 1979;132:777-82. [Crossref] [PubMed]
- Zhu K, Meng X, Qian J, Huang M, Li Z, Guan S, Jiang Z, Shan H. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis 2009;41:411-6. [Crossref] [PubMed]
- Sakai T, Shiraki K, Inoue H, Sugimoto K, Ohmori S, Murata K, Takase K, Nakano T. Complications of partial splenic embolization in cirrhotic patients. Dig Dis Sci 2002;47:388-91. [Crossref] [PubMed]
- Bezerra AS, D'Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol 2005;184:1510-3. [Crossref] [PubMed]
- Lokich J, Costello P. Splenic embolization to prevent dose limitation of cancer chemotherapy. AJR Am J Roentgenol 1983;140:159-61. [Crossref] [PubMed]
- Kauffman CR, Mahvash A, Kopetz S, Wolff RA, Ensor J, Wallace MJ. Partial splenic embolization for cancer patients with thrombocytopenia requiring systemic chemotherapy. Cancer 2008;112:2283-8. [Crossref] [PubMed]
- Winslow ER, Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery 2003;134:647-53. [Crossref] [PubMed]
- Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004;104:2623-34. [Crossref] [PubMed]
- Petermann A, Chabrot P, Cassagnes L, Dumousset E, Alfidja A, Gageanu C, Ravel A, Abergel A, Boyer L. Hypersplenism due to portal hypertension: retrospective evaluation of 17 patients treated by splenic embolization. Diagn Interv Imaging 2012;93:30-6. [Crossref] [PubMed]
- Sangro B, Bilbao I, Herrero I, Corella C, Longo J, Beloqui O, Ruiz J, Zozaya JM, Quiroga J, Prieto J. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology 1993;18:309-14. [Crossref] [PubMed]
- Harned RK 2nd, Thompson HR, Kumpe DA, Narkewicz MR, Sokol RJ. Partial splenic embolization in five children with hypersplenism: effects of reduced-volume embolization on efficacy and morbidity. Radiology 1998;209:803-6. [Crossref] [PubMed]
- Zhu K, Meng X, Li Z, Huang M, Guan S, Jiang Z, Shan H. Partial splenic embolization using polyvinyl alcohol particles for hypersplenism in cirrhosis: a prospective randomized study. Eur J Radiol 2008;66:100-6. [Crossref] [PubMed]
- Gonsalves CF, Mitchell EP, Brown DB. Management of hypersplenism by partial splenic embolization with ethylene vinyl alcohol copolymer. AJR Am J Roentgenol 2010;195:1241-4. [Crossref] [PubMed]
- Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009;7:250-63. [Crossref] [PubMed]
- Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg 2013;205:250-4. [Crossref] [PubMed]
- Cai M, Huang W, Lin C, Li Z, Qian J, Huang M, Zeng Z, Huang J, Shan H, Zhu K. Partial splenic embolization for thrombocytopenia in liver cirrhosis: predictive factors for platelet increment and risk factors for major complications. Eur Radiol 2016;26:370-80. [Crossref] [PubMed]
- He XH, Li WT, Peng WJ, Li GD, Wang SP, Xu LC. Total embolization of the main splenic artery as a supplemental treatment modality for hypersplenism. World J Gastroenterol 2011;17:2953-7. [Crossref] [PubMed]
- Luz JH, Luz PM, Marchiori E, Rodrigues LA, Gouveia HR, Martin HS, Faria IM, Souza RR, Gil RA, Palladino AM, Pimenta KB, de Souza HS. Partial splenic embolization to permit continuation of systemic chemotherapy. Cancer Med 2016;5:2715-20. [Crossref] [PubMed]
- Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: a review of the English language literature. J Vasc Interv Radiol 2007;18:463-81. [Crossref] [PubMed]
- Passhak M, Shachar SS, Ofer A, Beny A. Partial splenic embolization in the treatment of prolonged thrombocytopenia due to hypersplenism in metastatic cancer patients. Support Care Cancer 2018;26:3527-32. [Crossref] [PubMed]
- Heianna J, Muto O, Miyauchi T, Endo W, Togashi A, Azama K, Murayama S. Successful treatment continuation with a single mild partial splenic embolizationfor thrombocytopenia caused by oxaliplatin-based chemotherapy for advanced colon cancer. Indian J Gastroenterol 2016;35:245-7. [Crossref] [PubMed]
- Omer S, Zara O, Iacobescu C, Dina I. Partial splenic embolization for hypersplenism in cirrhotic patients. A case series. J Gastrointestin Liver Dis 2014;23:215-8. [Crossref] [PubMed]
- Griviau L, Chevallier O, Marcelin C, Nakai M, Pescatori L, Galland C, Midulla M, Falvo N, Loffroy R. Percutaneous ultrasound-guided balloon-assisted embolization of iatrogenic femoral artery pseudoaneurysms with Glubran®2 cyanoacrylate glue: safety, efficacy and outcomes. Quant Imaging Med Surg 2018;8:796-803. [Crossref] [PubMed]
- Jawhari R, Chevallier O, Falvo N, d'Athis P, Gehin S, Charles PE, Midulla M, Loffroy R. Outcomes of transcatheter arterial embolization with a modified N-butyl cyanoacrylate glue for spontaneous ilipsoas and rectus sheath hematomas in anticoagulated patients. J Vasc Interv Radiol 2018;29:210-7. [Crossref] [PubMed]
- Abdulmalak G, Chevallier O, Falvo N, Di Marco L, Bertaut A, Moulin B, Abi-Khalil C, Gehin S, Charles PE, Latournerie M, Midulla M, Loffroy R. Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: a single-center experience with 104 patients. Abdom Radiol (NY) 2018;43:723-33. [Crossref] [PubMed]
- Koizumi J, Itou C, Wray R, Myojin K, Hashimoto T, Nagata Y, Yamamuro H, Tsuji T, Ichikawa T, Shiraishi K, Kagawa T, Mine T, Watanabe N, Matsumae M, Janne d'Othée B. Partial splenic embolisation using n-butyl cyanoacrylate: intraprocedural evaluation by magnetic resonance imaging. Eur Radiol 2013;23:1429-42. [Crossref] [PubMed]


