Shear wave elastography evaluation of the median and tibial nerve in diabetic peripheral neuropathy
Introduction
Diabetic peripheral neuropathy (DPN) is one the major complications of diabetes mellitus (DM). The prevalence of peripheral neuropathy in type 2 diabetic patients is about 45%, and 54% to 59% in patients with type 1 diabetes (1). DPN has significant symptoms, typically presenting as a distal symmetric sensorimotor polyneuropathy, including neuropathic pain, numbness, burning sensation and so on. Furthermore, severe cases in the late stage may lead to serious outcomes, including neurogenic joints, ulceration, fractures, ischemic gangrene and even death (2,3). It is worth noting that the incidence of type 2 diabetes can occur between 4 and 7 years before clinical diagnosis, while adolescents may occasionally develop severe DPN in the first few months after onset of type 1 diabetes (4). Because of the high incidence of diabetic neuropathy, early detection and intervention of the occurrence of neuropathological symptoms are key. However, the early diagnosis and differential diagnosis of DPN remain difficult (5).
Nerve conduction study (NCS) and ultrasound are the commonly used methods for the detection of DPN, and both have advantages and disadvantages. NCS can predict disease in nerves by detecting their conduct velocity; however, this is time-consuming and invasive, and sometimes the action site cannot be induced. Moreover, it is easily affected by skin temperature and humidity (6). On the other hand, one study showed that high resolution ultrasound could image a lesion with relative ease and excellent resolution. With the improvement of technology, the clinical application of ultrasound in neuromuscular diseases has expanded rapidly (7,8). Ultrasound can provide important information of the cross-sectional area (CSA), the echo and the inner structure of nerve, and thus can reflect different degrees of DPN. A few previous studies have analyzed the relationship between the diabetic neuropathy and CSA of peripheral nerves on US (9,10). Studies on the use of US for polyneuropathy are mostly focused on the grey scale. However, research on the stiffness of the nerve is still limited. Shear wave elastography (SWE) is a promising technique widely applied in many organs such as the breast (11,12), thyroid (13,14), and the liver (15,16). In SWE, sound waves propagate in the form of a transverse wave. The acquisition of elastic information is based on the stimulation of ultrasound probes to tissues (17,18). The propagation velocity of shear wave is closely related to Young’s modulus formula. The hardness of the tissue can be reflected by assessing the velocity of shear wave propagation. The harder the tissue, the faster the shear wave travels (19). As a good adjunctive diagnostic tool to conventional US, SWE may provide more quantitative evaluation of tissue, which could improve the diagnostic performance of US.
So far, we are aware of only a few reports on SWE in the evaluation of DPN. In this study, we aimed to investigate the usefulness of SWE for the diagnosis of polyneuropathy in diabetic patients by investigating the median and tibial nerves.
Methods
This study was approved by the Ethics Committee of West China Hospital, Sichuan University, and performed in accordance with the Helsinki Declaration of 2013. All patients and volunteers provided signed informed consent for the scientific acquisition and analysis of image data at the time of examination.
Participants
The inclusion criteria for the DM group were as follows: diabetes was diagnosed by using the revised American Diabetes Association criteria, which include a fasting plasma glucose level of at least 7.1 mmol/L and a 2-hour postprandial plasma glucose level of at least 11.1 mmol/L.
The inclusion criteria for the DPN group were as follows: type 2 DM and confirmed diagnosis of DPN. The clinical criteria for the diagnosis of DPN required the presence of more than one symptom (e.g., tingling, numbness, burning sensation, ataxia) or the persistence and progression of the distal symmetric neuropathic pattern (e.g., abnormal knee joint or ankle reflexes, temperature, touch, pain and vibration sensation). The patients were assessed clinically using neuropathy symptom score (NSS) and neuropathy disability score (NDS). The clinical criteria for the diagnosis of DPN was based on the method described by Young et al. (20). Routine NCS was performed in the DM and DPN patients. Motor nerve conduction velocities of median and tibial nerves were recorded.
The control group participates came from the population without DM. The NCS test was not performed in healthy volunteers.
Exclusion criteria were the presence of type 1 DM; and a known history of polyneuropathy from other etiologic causes, such as hereditary, alcoholic, metabolic, inflammatory, or toxic factors.
Forty diabetic patients who were confirmed with DPN, forty diabetic patients who were confirmed without DPN, and forty healthy volunteers who visited West China Hospital from November 2016 to July 2017 were enrolled in this study.
Sonographic examinations
All US examinations were performed by using a 4–15 MHz linear-array transducer (Aixplorer; SuperSonic Imagine, Les Jardins de la Duranne, Aix en Provence, France). The subjects were lying in the supine position, keeping upper and lower limbs relaxed. The position of all participants was standardized to avoid any ankle and forearm movement that might have infected soft-tissue stiffness. The nerve was scanned in cross section, and the CSA was measured at 3 cm above the medial malleolus of the tibial nerve, avoiding the branch of tibial nerve. Then, the median nerve was measured at the midpoint of the forearm.
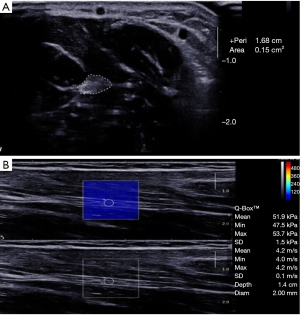
SWE were performed gently, and sufficient ultrasonic coupling agent was used to make the probe touch lightly on the skin surface to avoid the compression effect of the sensor. First, the tibial nerve and median nerve were identified on the transverse imaging plane; then the probe was rotated 90 degrees to obtain the longitudinal imaging, which is the plane parallel to the nerve fiber direction. The region of interest (ROI) size was fixed to 2 mm in all cases. Note that the circular ROI should be placed inside the nerve epineurium border. The stiffness (in m/s) of the ROI was then generated automatically based on the integrated SWE software.
The entire study group was independently completed by two ultrasound physicians, both with 5-year experience. The CSA and SWE of nerves were measured three times at each site to increase reproducibility, with the average value being chosen for the following analysis. The observers were blinded to both images obtained at previous examinations and the results of previous examinations, including the patient’s clinical history and NCS results. Observer 1 repeated elastographic measurement 1 week after the initial interpretation.
Statistical analysis
SPSS (version 22.0) software was used for the analysis of data. The Anderson-Darling normality test was used for evaluation of normal distribution. Mann-Whitney U test and the independent t-test were performed to compare the data of baseline characteristics depending on the distribution. Continuous variables were expressed as means ± standard deviations. The t-test was performed to compare CSA and SWE of the nerve in the DPN, DM and control groups. P<0.05 values were accepted as statistically significant.
The receiver operating characteristic (ROC) curves were depicted to determine the optimal possible cut-off values of nerve CSA and SWE for the diagnosis of DPN. Sensitivity, specificity, area under the curve (AUC) of ROC were calculated.
Results
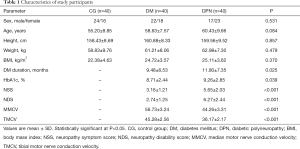
The baseline demographic data of the control, DM and DPN groups are displayed in Table 1. No significant differences in the sex ratio, age, height, weight and BMI were found in the three groups. The HbA1c levels in the DPN group were higher than the DM group (P=0.039), and the courses of disease in the DPN group were longer than the DM group (P=0.025). The scores of NSS and NDS in the DPN group were higher than the DM group (P<0.001). The motor nerve conduction velocity of median nerve and tibial nerve in the DPN group was lower than that in the DM group (P<0.001).
Full table
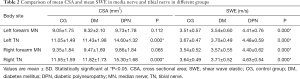
Bilateral comparisons showed that there was no significant difference in the CSA of the left and right median nerve and tibial nerve in DPN group (P>0.05). There was also no significant difference in the CSA of the left and right median nerves and tibial nerves in the diabetic group (P>0.05) and the control group (P>0.05).
There was no significant difference of the median nerve CSA among the control group, the DM group and DPN group (P>0.05, Table 2). In regard to tibial nerve CSA, the DM group was not significantly different from the control group (P>0.05), although it was significantly different from the DPN group (P<0.001).
Full table
Bilateral analysis showed that there was no significant difference in nerve stiffness between the left and right median nerves and tibial nerves in DPN patients (P>0.05). There was also no significant difference in nerve stiffness between the left and right median nerves and tibial nerves in the diabetic group (P>0.05) and the control group (P>0.05). The stiffness of the median nerve and tibial nerve in each one side also had no significant difference in patients with DPN (P>0.05). Median nerve (Figure 1A,B) and tibial nerve (Figure 2A,B) of the DPN group were significantly stiffer than those of the DM group and control subjects (P<0.001). The elasticity of the median nerve and tibial nerve between the DM group and control group had no significant difference.

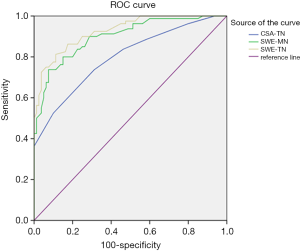
The optimal cut-off value of tibial nerve CSA for diagnosis of DPN was 12.5 mm2, with a sensitivity of 73.8%, and a specificity of 68.7%. The optimal cut-off value of tibial nerve SWE for diagnosis of DPN was 4.11 m/s, with a sensitivity of 81.3%, and a specificity of 88.7%. The AUC was 0.927 (95% CI: 0.890–0.964). The optimal cut-off value of median nerve SWE for diagnosis of DPN was 4.06 m/s, with a sensitivity of 80%, and a specificity of 85%. The AUC was 0.899 (95% CI: 0.853–0.946). The AUC of the SWE (median nerve and tibial nerve) to diagnose diabetic neuropathy was significantly greater than that of tibial nerve CSA (0.798; 95% CI: 0.731–0.866) (Figure 3), which suggests that SWE was a better technique for characterizing the presence of diabetic neuropathy. It can be evaluated as an effective adjunctive method in diagnosis of diabetic neuropathy.
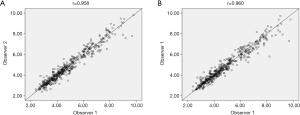
There was excellent inter- and intra-observer consistency of the SWE. The consistency value for the elasticity was 0.958 and 0.960 (Figure 4).
Discussion
DPN is one of the most common complications of diabetes, which is characterized by symmetric sensory abnormalities of the distal extremities. High resolution ultrasound is a cheap, noninvasive examination method (21,22). It has unique advantages to the morphological description of neuropathy, which makes it possible to visualize the location and range of the lesion (23). It is widely used in the detection of neuropathy. However, few studies have been conducted to assess the elasticity of DPN. Moreover, these studies are limited to assessing the elasticity of the tibial nerve in the lower extremities, while DPN is a multiple neuropathy in the distal part of the extremities. In this study, the tibial nerve in the lower extremities and the median nerve of the middle forearm were selected as the targeted nerve. We chose a location approximately 3 cm proximal to the medial malleolus as the measurement site of tibial nerves to avoid the bifurcation of the tibial nerve based on the study of Riazi, which reported that a measurement at 3 cm above the medial malleolus had an optimal threshold value for identification of DPN (24). The midpoint of the forearm was chosen for the assessment of the median nerve. DPN is a distal symmetrical sensorimotor abnormality. Normally, the measuring point should be selected as the distal part of the median nerve, but the shear wave elastic imaging of the median nerve at the carpal canal is easily affected by the transverse ligaments of the wrist. In previous studies, ultrasound has been commonly used in the diagnosis and evaluation of carpal tunnel syndrome. In DPN, the thickening of the median nerve in the wrist may also form carpal tunnel syndrome. To identify carpal tunnel syndrome caused by DPN or other reasons, the forearm midpoint was selected as the measurement part of the median nerve. DPN is also known as a length-dependent polyneuropathy. Moon et al. (25) found that the CSA of the median nerve at the palm to distal forearm level in the DPN group was significantly greater than those of the DM group and control group. The results once again confirmed those of previous studies (26), and further demonstrated that the CSA of the median nerve altered not only focusing around the wrist, but also involving the proximal nerve.
High-resolution ultrasound is widely used in the diagnosis of a variety of peripheral nerve diseases. Normal peripheral nerves have a characteristic echotexture (27). High-resolution ultrasounds can clearly depict peripheral nerve size, spaces occupied by lesions, and anatomic variants along the entire length of the normative nerve. Ultrasonography patterns correlate well with histological structures. In previous studies, ultrasound diagnosis of most peripheral neuropathy was based on CSA measurement. The study of Hobson-Webb et al. (28), which reported on multiple parts of the sural and fibular nerves in patients with DPN and a control group, found that there was no statistical difference between the two groups in the nerve CSA, diameter and echogenicity. However, the study of diabetic patients with neuropathic symptoms carried out by Pitarokoili et al. (29) demonstrated that the CSA of the peripheral nerve in both the compression and non-compression sites could be increased. According to the study of Kelle et al. (30), CSAs of the sciatic, tibial and median nerves in DPN patients were larger than those in healthy individuals. In the present study, the tibial nerve CSA was significantly greater in diabetic patients in the DPN group than in healthy controls and in the diabetic patients without DPN group. However, the CSA of the median nerve was not different among the three groups. The reason for this finding may be that the measurement sites of the median nerve was in the middle forearm in this study, which is not the common compressive site of the median nerve.
SWE as a noninvasive evaluation technique based on ultrasound, can reflect the elasticity of tissue through the propagation of shear waves. The velocity of shear wave in the region of interest rises with the increase of tissue stiffness (31). Ultrasound elastography has been applied to the musculoskeletal system in parts like the Achilles tendon (32), plantar fasciitis (33) and so on, which enables clinicians to assess the elasticity of tissue in situ. In our study, we evaluated the median nerve and tibial nerve stiffness of the DPN with shear wave velocity. The inter-observer and intra-observer reproducibility of the SWE with DPN was found to be excellent in our study. The patients with DPN presented a higher nerve stiffness and a greater CSA on the tibial nerve compared with that of the DM group and control group. The stiffness of median nerve in the DPN group was higher than the other two groups, while the CSA showed no significant difference. This indicates that the diagnosis value of SWE was better than CSA even in the section of the proximal limb and non-nerve entrapment point. The pathophysiology of DPN was believed to result from a process where the edema within the nerve fascicle increases intraneural pressure, making the nerve stiffer. The increased stiffness results in further compression of the microvasculature and the reduction of blood flow (34,35). These changes have been shown to play the most important etiologic role in the production of DPN (36), and these changes can lead to focal demyelination and axonal degeneration with the fibrotic response (37). This may then result in the proliferation of scar tissue and an increase in the speed of shear wave propagation in the median nerve (38). Dikici et al. found that the tibial nerves of diabetic patients with DPN were significantly stiffer than those of diabetic patients without DPN and healthy control subjects (39). By using strain elastography, Ishibashi et al. also found that the elasticity of the tibial nerve in patients with diabetic neuropathy was stiff when compared with controls (40).
To investigate whether using CSA or SWE could accurately diagnose DPN, we compared the sensitivity and specificity of different modalities by using ROC curve analysis. Both sensitivity and specificity of SWE were higher than that of CSA. SWE had certain advantages because it was operator-independent, reproducible, and quantitative. SWE provided more direct and quantitative evaluation of tissue stiffness, and it did not depend on operator compression of the probe. In the study by Kang et al. the optimal cut-off values of median nerve CSA for diagnosis of DPN were 9.4 mm2 at the carpal tunnel, and 8.9 mm2 at the middle part of the humerus. The sensitivities were 80% and 65%, while specificities were 70% and 60%, respectively (41). By using strain elastography, Ishibashi et al. recently reported that the AUC for the elasticity (0.829) was significantly greater than that for the CSA (0.612) of the tibial nerve (40). Dikici et al. found that a cut-off value of 51.0 kPa at 4 cm proximal to the medial malleolus revealed a sensitivity of 90% and a specificity of 85.0% (39). In this study, both the sensitivity and specificity of SWE were higher than that of CSA when diagnosing DPN, which is mostly in line with the published research.
DPN is a multiple neuropathy of the distal extremities. Currently, studies on DPN ultrasound elastography are mostly limited to the evaluation of the elasticity of lower limb nerves. The innovation of this study lies in that it not only evaluates the tibial nerve of the lower extremity by SWE, but also selects the median nerve of the upper extremity for the analysis. The elastic values of the median nerve and tibial nerve in DPN patients were also compared. The results showed that there was no statistically significant difference between them. In addition, when the median nerve was detected, the location selected in this study was in the middle of the forearm, which was different from the previous examinations where the wrist was most commonly selected as the measurement site. The results also showed that the nerve stiffness of the DPN group was higher than that of the DM group and control group, which prove that DPN was changed in the proximal nerve.
There still exist several limitations in our study. First, DPN is a multiple peripheral nerve disease; although we chose the median nerve and tibial nerve to examine, many more peripheral nerves and multiple parts of the same nerve also should be measured, such as the following regions: for the median nerve, at the wrist 2 cm proximal to the wrist crease at the elbow; for the tibial nerve, at the popliteal fossa and so on. Additionally, this study did not investigate the relationship of the nerve stiffness and the degree of diabetic neuropathy in detail. For this purpose, longitudinal studies are needed to show the temporal changes on nerve stiffness. Finally, we could not confirm the change of DPN stiffness from histopathology because nerve biopsies were not performed,
In conclusion, the SWE of nerves in patients with diabetes neuropathy was stiffer than that in patients with diabetes or in the control group. In this study, the tibial nerve CSA was significantly higher in diabetic patients with DPN, while no such difference was observed between the DM group and control group. The CSA of the median nerve in the three groups was not significantly different. Meanwhile, the stiffness of the median and tibial nerves was significantly higher in the DPN group. Overall, these findings suggest that SWE-based stiffness measurement of the nerve was a better method than CSA, and it can thus be used as another effective assistant method in the diagnosis of DPN.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (81671696).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of West China Hospital, Sichuan University, and performed in accordance with the Helsinki Declaration of 2013. All patients and volunteers provided signed informed consent for the scientific acquisition and analysis of image data at the time of examination.
References
- Zilliox L, Russell JW. Treatment of diabetic sensory polyneuropathy. Curr Treat Options Neurol 2011;13:143-59. [Crossref] [PubMed]
- Turns M. The diabetic foot: an overview of assessment and complications. Br J Nurs 2011;20:S19-25. [Crossref] [PubMed]
- Khazai NB, Beck GR Jr, Umpierrez GE. Diabetes and fractures: an overshadowed association. Curr Opin Endocrinol Diabetes Obes 2009;16:435-45. [Crossref] [PubMed]
- Said G. Diabetic neuropathy. Handb Clin Neurol 2013;115:579-89. [Crossref] [PubMed]
- Dyck PJ1. Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ, O'Brien PC; Cl vs. NPhys Trial Investigators, Albers JW, Andersen H, Bolton CF, England JD, Klein CJ, Llewelyn JG, Mauermann ML, Russell JW, Singer W, Smith AG, Tesfaye S, Vella A. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010;42:157-64.
- Colak A, Kutlay M, Pekkafali Z, Saraçoglu M, Demircan N, Simşek H, Akin ON, Kibici K. Use of sonography in carpal tunnel syndrome surgery: a prospective study. Neurol Med Chir (Tokyo) 2007;47:109-15. [Crossref] [PubMed]
- Kerasnoudis A, Tsivgoulis G. Nerve ultrasound in peripheral neuropathies: a review. J Neuroimaging 2015;25:528-38. [Crossref] [PubMed]
- Suk JI, Walker FO, Cartwright MS. Ultrasonography of peripheral nerves. Curr Neurol Neurosci Rep 2013;13:328. [Crossref] [PubMed]
- Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, Takeda J, Seishima M, Matsuoka T. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med 2010;29:697-708. [Crossref] [PubMed]
- Lee D, Dauphinee DM. Morphological and functional changes in the diabetic peripheral nerve: using diagnostic ultrasound and neurosensory testing to select candidates for nerve decompression. J Am Podiatr Med Assoc 2005;95:433-7. [Crossref] [PubMed]
- Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, Youk JH, Son EJ, Choi SH, Kook SH, Chung J, Cha ES, Park JS, Jung HK, Ko KH, Choi HY, Ryu EB, Moon WK. Korean Breast Elastography Study Group. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography 2014;33:3-10. [Crossref] [PubMed]
- Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography. 2014;33:34-9. [Crossref] [PubMed]
- Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab 2010;95:5281-8. [Crossref] [PubMed]
- Kim H, Kim JA, Son EJ, Youk JH. Quantitative assessment of shear-wave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. Eur Radiol 2013;23:2532-7. [Crossref] [PubMed]
- Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, Tripon S, Terris B, Mallet V, Sogni P, Tanter M, Pol S. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol 2015;62:317-24. [Crossref] [PubMed]
- Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, Lee KB, Han JK, Choi BI. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 2014;273:772-82. [Crossref] [PubMed]
- Sarvazyan AP, Urban MW, Greenleaf JF. Acoustic waves in medical imaging and diagnostics. Ultrasound Med Biol 2013;39:1133-46. [Crossref] [PubMed]
- Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013;34:169-84. [Crossref] [PubMed]
- Engel AJ, Bashford GR. A new method for shear wave speed estimation in shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2015;62:2106-14. [Crossref] [PubMed]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom Hospital Clinic Population. Diabetologia 1993;36:150-4. [Crossref] [PubMed]
- Liang HY, Liang XW, Chen ZY, Tan XH, Yang HH, Liao JY, Cai K, Yu JS. Ultrasound in neonatal lung disease. Quant Imaging Med Surg 2018;8:535-46. [Crossref] [PubMed]
- Wang L, Xiang X, Tang Y, Yang Y, Qiu L. Sonographic appearance of fluid in peripheral joints and bursae of healthy asymptomatic Chinese population. Quant Imaging Med Surg 2018;8:781-7. [Crossref] [PubMed]
- Kopf H, Loizides A, Mostbeck GH, Gruber H. Diagnostic sonography of peripheral nerves: indications, examination techniques and pathological findings. Ultraschall Med 2011;32:242-63. [Crossref] [PubMed]
- Riazi S, Bril V, Perkins BA, Abbas S, Chan VW, Ngo M, Lovblom LE, El-Beheiry H, Brull R. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabetes Care 2012;35:2575-9. [Crossref] [PubMed]
- Moon HI, Kwon HK, Kim L, Lee HJ, Lee HJ. Ultrasonography of palm to elbow segment of median nerve in different degrees of diabetic polyneuropathy. Clin Neurophysiol 2014;125:844-8. [Crossref] [PubMed]
- Watanabe T, Ito H, Morita A, Uno Y, Nishimura T, Kawase H, Kato Y, Matsuoka T, Takeda J, Seishima M. Sonographic evaluation of the median nerve in diabetic patients: Comparison with nerve conduction studies. J Ultrasound Med 2009;28:727-34. [Crossref] [PubMed]
- Chen J, Wu S, Ren J. Ultrasonographic reference values for assessing normal radial nerve ultrasonography in the normal population. Neural Regen Res 2014;9:1844-9. [Crossref] [PubMed]
- Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve 2013;47:379-84. [Crossref] [PubMed]
- Pitarokoili K, Kerasnoudis A, Behrendt V, Labedi A, Ayzenberg I, Gold R, Yoon MS. Facing the diagnostic challenge: Nerve ultrasound in diabetic patients with neuropathic symptoms. Muscle Nerve 2016;54:18-24. [Crossref] [PubMed]
- Kelle B, Evran M, Balli T, Yavuz F. Diabetic peripheral neuropathy: Correlation between nerve cross-sectional area on ultrasound and clinical features. J Back Musculoskelet Rehabil 2016;29:717-22. [Crossref] [PubMed]
- Chen S, Urban MW, Pislaru C, Kinnick R, Zheng Y, Yao A, Greenleaf JF. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:55-62. [Crossref] [PubMed]
- Frankewycz B, Penz A, Weber J, da Silva NP, Freimoser F, Bell R, Nerlich M, Jung EM, Docheva D, Pfeifer CG. Achilles tendon elastic properties remain decreased in long term after rupture. Knee Surg Sports Traumatol Arthrosc 2018;26:2080-7. [Crossref] [PubMed]
- Putz FJ, Hautmann MG, Banas MC, Jung EM. Investigation of the acute plantar fasciitis with contrast-enhanced ultrasound and shear wave elastography - first results. Clin Hemorheol Microcirc 2017;67:415-23. [Crossref] [PubMed]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736-43. [Crossref] [PubMed]
- Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain 1984;107:935-50. [Crossref] [PubMed]
- Dellon AL. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy: update 2006. Acta Neurochir Suppl 2007;100:149-51. [Crossref] [PubMed]
- Ibrahim I, Khan WS, Goddard N, Smitham P. Carpal tunnel syndrome: a review of the recent literature. Open Orthop J 2012;6:69-76. [Crossref] [PubMed]
- Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen 2011;19:117-33. [Crossref] [PubMed]
- Dikici AS, Ustabasioglu FE, Delil S, Nalbantoglu M, Korkmaz B, Bakan S, Kula O, Uzun N, Mihmanli I, Kantarci F. Evaluation of the Tibial Nerve with Shear-Wave Elastography: A Potential Sonographic Method for the Diagnosis of Diabetic Peripheral Neuropathy. Radiology 2017;282:494-501. [Crossref] [PubMed]
- Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes. J Diabetes Investig 2016;7:404-12. [Crossref] [PubMed]
- Kang S, Kim SH, Yang SN, Yoon JS. Sonographic features of peripheral nerves at multiple sites in patients with diabetic polyneuropathy. J Diabetes Complications 2016;30:518-23. [Crossref] [PubMed]