
Transcatheter arterial embolization of a splenic artery aneurysm with N-butyl cyanoacrylate/lipiodol/ethanol mixture with coil-assisted sandwich technique
Introduction
Splenic artery aneurysm (SAA) is the most common visceral artery aneurysm, with a reported prevalence of 0.8% on arteriography and 0.04–0.10% at autopsy (1). Abbas et al. (2) reported that asymptomatic SAAs of >2 cm in size have an increased risk of rupture. SAAs are both more frequent and larger in adults with chronic liver disease and portal hypertension (3), and SAA rupture is associated with a higher mortality rate in patients with portal hypertension than in other patients (3). Percutaneous transcatheter embolization of SAAs with coils is widely accepted as the first line of treatment because it is safe, has a low mortality rate, and provides adequate short- and long-term results (4).
However, embolization of SAAs usually requires many coils when the isolation packing technique is used. Incomplete embolization carries the risk of coil compaction and the recanalization of SAA (5). To overcome these limitations, we describe a new technique of endovascular liquid packing with balloon occlusion for SAA, using an n-butyl cyanoacrylate (NBCA; Histoacryl, B. Braun, Melsungen, Germany)/lipiodol (Ultra-Fluide®, Guerbet, Roissy, France)/ethanol (Fuso Pharmaceutical Industries Ltd, Osaka, Japan) (NLE) mixture, in which the addition of ethanol confers low adhesive properties on the NBCA glue (6-8).
Case presentation
Our Institutional Review Board approved this study. Patient consent for the publication of this case report was obtained. Computed tomography (CT) performed as follow-up revealed an SAA in a 59-year-old man suffering from cirrhosis, esophageal varix, and splenomegaly. Hypersplenism was also present. The aneurysm was monitored for 4 years. The patient was admitted to our hospital for a therapeutic procedure when the diameter of the aneurysm had increased to 20 mm. Because of coexisting chronic liver disease, a less invasive endovascular procedure was the method of choice. We explained to the patient that embolization of the SAA would probably cause splenic infarction, but could also reduce his splenomegaly and increase his platelet count. The patient gave his written informed consent.
A 5 French (Fr.) sheath (Terumo, Tokyo, Japan) was inserted into the right femoral artery and a 4 Fr. sheath (Terumo) was inserted into the left femoral artery. A 4 Fr. RC2 catheter (Rosch celiac, Medikit, Tokyo, Japan) was inserted into the splenic artery via the left femoral artery, and celiac arteriography and splenic arteriography were performed. Arteriography revealed a true solitary aneurysm located in the splenic hilum, with one distal splenic arterial branch (Figure 1A). A 5 Fr. 9 mm diameter balloon catheter (Selecon MP Catheter; Terumo) was inserted into the splenic artery via the right femoral artery. A 2.8 Fr. microcatheter (Carnelian; Tokai Medical Products, Aichi, Japan) was inserted through the balloon catheter into the distal splenic arterial branch of the aneurysmal sac. The distal branch was embolized with six detachable coils (Target XL®, Stryker Neurovascular, Fremont, CA, USA and Ruby® Coil, Penumbra Inc., Alameda, CA, USA) under balloon occlusion to prevent coil migration. Following embolization of the distal branch, the microcatheter was removed and the balloon catheter was placed in the proximal portion of the SAA. The embolic material was prepared by mixing NBCA, lipiodol, and ethanol in a 1:4:1 ratio. Before the injection of the NLE, the balloon catheter was thoroughly flushed with 5% nonionic dextrose solution to prevent premature polymerization. The NLE (3.5 mL) was slowly injected via the balloon catheter under balloon occlusion until it entirely filled the aneurysmal sac. After the aneurysm sac was completely filled, balloon occlusion was maintained for 10 min to allow complete thrombogenesis (Figure 1B).
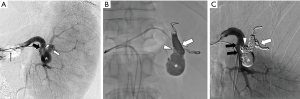
After the procedure, the balloon was deflated and the catheter was easily removed without adhesion to the embolic material. Finally, a microcatheter was advanced through the 4 Fr. catheter in the left femoral artery, into the splenic artery proximal to the aneurysmal sac, and the proximal portion of the aneurysm was embolized with four detachable coils (Target XL®, Stryker Neurovascular). Postembolization splenic arteriography showed the complete embolization of the aneurysm and patency of the arterial branch to the lower splenic pole (Figure 1C). Celiac angiography after the procedure showed no “back-door” flow to the aneurysm sac.
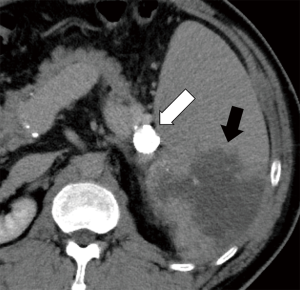
Enhanced CT performed 3 days after the procedure showed a dense accumulation of NLE within the aneurysm (Figure 2), and a partial splenic infarction, which was predicted. The patient had a slight fever, but no abdominal pain, and after 4 days was discharged from hospital without major complications.
Discussion
Transcatheter coil embolization is currently considered the treatment of choice for SAAs (4,5). Splenic infarction is the most frequent postprocedural complication, but most instances resolve without sequelae (9). Visceral aneurysm embolization can usually be performed with the isolation and packing technique. However, this procedure requires many coils to avoid coil compaction (5). Therefore, we used NLE to reduce the number of coils required. It has been reported that NLE is less adhesive than NBCA (6-8). Tanaka et al. reported that NLE doesn’t adhere to the balloon catheter and showed the feasibility of packing a wide-neck aneurysm (8). The major concern with NBCA is its adhesion to the catheter and early polymerization within the catheter and the proximal portion of the aneurysm, leading to incomplete embolization of the aneurysm. However, NLE can be injected slowly and in a controlled manner, without its adhesion to the catheter or the need for its rapid removal (6,7).
The optimal ration and amount of NLE might vary according to the size of the aneurysm and its flow. Based on our experience, our mixing ratio (NLE =1:4:1) enabled us to achieve complete packing of the SAA without regurgitation. We believe that this mixing ratio was appropriate for SAA embolization, considering adhesion of NLE glue and safety.
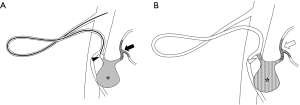
In this case, NLE was sandwiched between the proximal and distal coils using a technique we call “liquid packing with a coil-assisted sandwich technique” (Figure 3). This method may increase the durability of NLE and promote thrombogenesis in the aneurysm. NLE may also exert a synergic effect with ethanol, promoting sac occlusion and the destruction of the endothelial lining, thus preventing sac recanalization (8). Consequently, the number of coils required is reduced, which shortens the procedural time.
A limitation of this procedure is that the optimal ratio and amount of NLE might vary according to the size of the aneurysm and its inflow. Another limitation is that this technique should be avoided for SAAs in the middle segment of the splenic artery, because it has been reported that pancreatitis complications mainly occur when glue embolization occurs in the pancreatic arteries branching in the middle segment of the splenic artery (10).
Conclusions
Transcatheter arterial embolization using liquid packing with the coil-assisted sandwich technique for SAA reduces the number of coils that are normally used and is a safe and feasible treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Berceli SA. Hepatic and splenic artery aneurysms. Semin Vasc Surg 2005;18:196-201. [Crossref] [PubMed]
- Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, Pairolero PC, Hallett JW, Bower TC, Panneton JM, Cherry KJ. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002;16:442-9. [Crossref] [PubMed]
- Evans HM, Sharif K, Brown RM, Platt C, Crisp WJ, Kelly DA. Fatal and life threatening rupture of splenic artery aneurysms in children with portal hypertension. Pediatr Transplant 2004;8:192-5. [Crossref] [PubMed]
- Loffroy R, Guiu B, Cercueil JP, Lepage C, Cheynel N, Steinmetz E, Ricolfi F, Krausé D. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short- and long-term results. Ann Vasc Surg 2008;22:618-26. [Crossref] [PubMed]
- Yasumoto T, Osuga K, Yamamoto H, Ono Y, Masada M, Mikami K, Kanamori D, Nakamura M, Tanaka K, Nakazawa T, Higashihara H, Maeda N, Tomiyama N. Long-term outcomes of coil packing for visceral aneurysms: correlation between packing density and incidence of coil compaction or recanalization. J Vasc Interv Radiol 2013;24:1798-807. [Crossref] [PubMed]
- Kawai N, Sato M, Minamiguchi H, Ikoma A, Sanda H, Nakata K, Tanaka F, Nakai M, Sonomura T. Basic study of a mixture of N-butyl cyanoacrylate, ethanol, and lipiodol as a new embolic material. J Vasc Interv Radiol 2012;23:1516-21. [Crossref] [PubMed]
- Nakai M, Ikoma A, Sato M, Sato H, Nishimura Y, Okamura Y. Prophylactic intraoperative embolization of abdominal aortic aneurysm sacs using N-butyl cyanoacrylate/lipiodol/ethanol mixture with proximal neck aortic balloon occlusion during endovascular abdominal aortic repair. J Vasc Interv Radiol 2016;27:954-60. [Crossref] [PubMed]
- Tanaka F, Kawai N, Sato M, Minamiguchi H, Nakai M, Nakata K, Sanda H, Sonomura T. Balloon-assisted packing of wide-neck aneurysms with a mixture of n-butyl cyanoacrylate, Lipiodol, and ethanol: an experimental study. Jpn J Radiol 2015;33:517-22. [Crossref] [PubMed]
- Yoon T, Kwon T, Kwon H, Han Y, Cho Y. Transcatheter arterial embolization of splenic artery aneurysms: A single-center experience. Vasc Specialist Int 2014;30:120-4. [Crossref] [PubMed]
- Tokuda T, Tanigawa N, Kariya S, Komemushi A, Nomura M, Suzuki S, Nakatani M, Yagi R, Sawada S. Pancreatitis after transcatheter embolization of a splenic aneurysm. Jpn J Radiol 2010;28:239-42. [Crossref] [PubMed]


