
Diagnosis of functional paraganglioma by ultrasonographer squeezing the retroperitoneal tumor and measuring the change of blood pressure: A case presentation
Introduction
Paraganglioma (PGL), also known as chemoreceptor tumor, can occur in various parts of the body, with the most common one in the adrenal gland called pheochromocytoma (PHEO). As PGL arise within the sympathetic nervous system which originates from the neural crest (1), some PGLs have the ability to secrete catecholamine and are characterized by symptomatic hypertension (2,3). Inspired by the fact that PGL patients’ blood pressure rises when the surgeons touch the tumor during operation, a chance idea occurred to us to squeeze the tumor during ultrasound examination to see if there is a significant fluctuation in blood pressure, and to determine whether the tumor has endocrine function. Our experience in one case is described below.
Case presentation
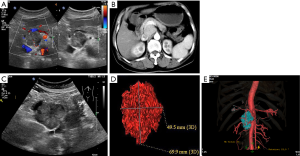
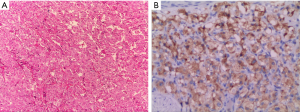
A 54-year-old woman, with diabetes for 7 years and hypertension for 2 years, was found to have a tumor of about 35 mm × 32 mm in abdominal cavity during a health check in December 2015 (Figure 1A). The tumor was located behind the pancreas and was closely related to the pancreas, so it was considered as a pseudocyst of the pancreas. She felt no discomfort and decided to follow-up it regularly. In March 2016, the patients were re-examined with enhanced CT, and the tumor size was 42 mm × 33 mm (Figure 1B), and still considered as a pancreatic pseudocyst. In July 2017, the patient underwent ultrasound examination again. The size of tumor was measured about 60 mm × 50 mm (Figure 1C), with sphere shape and part of the boundary was unclear. Its interior was dominated by low echo and the blood flow was not abundant. PGL was suspected, and the authors tried to test whether the tumor had endocrine function. An electronic sphygmomanometer was placed around this patient’s left elbow joint, and the tumor was pressed with the moderate pressure during the examination. The brachial artery blood pressure was measured before and after compression respectively. The blood pressure was 135/75 mmHg before pressing the tumor, and it rose to 196/105 mmHg after pressing the tumor. According to this observation, the tumor was diagnosed as a functional PGL. The patient underwent a CT scan the following day. The size of the tumor was measured about 70 mm × 50 mm (Figure 1D,E) and was significantly enhanced in arterial phase, and had no obvious invasion of surrounding blood vessels. After adequate preoperative preparation, the tumor was removed surgically and confirmed that touching the tumor caused blood pressure fluctuations during the operation. The pathology confirmed the tumor was paraganglioma (Figure 2), and immunohistochemical analysis showed that CgA, NSE, S-100, Syn and Vim were all positive.
Discussion
Clinically conventional ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are often used to detect and diagnose tumors. Although CT and MRI generally perform better than conventional US, a systematic review showed that PGL/PHEO with atypical presentations may be poorly characterized by CT or MRI (4). One of concerns about PGL is whether the tumor is benign or malignant, some nuclear imaging methods can predict the tumor invasiveness. Brito et al. (5) concluded functional imaging is of limited value in the diagnosis of PGL, but has certain significance in differentiating benign and malignant tumors through a summary analysis of 32 studies published from 1983 to 2012 that evaluated nuclear imaging in patients with pathologically proven PGL. In malignant PGL, 18F-FDA-PET had excellent performance at identifying lesions which not detected in CT/MRI. In a study of 28 PGLs/PHEOs (6), the uptake intensity ratio of 131I-MIBG was significantly higher in malignant lesions compared to benign ones (P<0.01). PGLs often were highly vascularized tumors in contrast-enhanced ultrasound (CEUS), and the contrast agent appears inside the tumor very early in the arterial phase (7); for the diagnosis of malignant adrenal mass, the sensitivity and specificity of CEUS have been reported to be 100% and 67%, CEUS and MRI/CT were congruent concerning the characterization of malignant versus benign adrenal masses (8). Considering the deficiency of CT/MRI/US in differentiating benign and malignant PGL, the stratification by biochemical, immunohistochemical, and molecular profiling is also applied clinically for supplementary information (9).
Another concern for this type of tumors is whether the tumor has endocrine function as this is for preoperative preparation. Nuclear imaging may not be very useful in this aspect (10). The levels of catecholamines and metabolites in plasma or urine are useful to assess endocrine function, but some PGLs may present negative results as catecholamine is secreted intermittently. When the tumor was touched and squeezed during surgery, the tumor can releases catecholamine. In order to reduce the potential impact of catecholamine on blood pressure, α-receptor blockers phentolamin and β-receptor blockers are necessary for patients before surgery regardless of whether PGL secretes catecholamine or not. King et al. (11) found that the patients with catecholamine-secreting PGL/PEHO may cause exercise-induced nausea and vomiting, likely due to the increase of catecholamine secretion caused by compression of tumors in exercise. In our case, a test was used to observe the fluctuation of blood pressure by ultrasonographer, which provided sufficient evidences for operation preparation. In addition, in order to know the effect of drug control preoperative preparation, ultrasonographer can try again to squeeze tumor and monitor blood pressure rise. Unfortunately, the patient did not measure metanephrine (MN) of plasma and urine, nor did to assess the fluctuation of blood pressure again before operation. Report of whether PGL is catecholamine-secreting during imaging procedure is rare, and to our knowledge this case was a first attempt diagnosis by ultrasonographer. The strength of squeezing tumor was an important factor of blood pressure fluctuation, so we used the method of repeated intermittent compression and measure blood pressure after stopping compression, and halted the trial when there was a marked increase in blood pressure or when the patient felt dizzy.
Acknowledgements
Pathological pictures provided by doctor Ying Zhu (Department of Pathology, Dongguan People’s Hospital Affiliated to Southern Medical University); the grammar amendment of the manuscript was supported by Prof. Wang Yixiang, Prince of Wales Hospital, the Chinese University of Hong Kong.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient, and the study protocol was approved by the institution’s human research committee.
References
- Kavinga Gunawardane PT, Grossman A. Phaeochromocytoma and Paraganglioma. Adv Exp Med Biol 2017;956:239-59. [Crossref] [PubMed]
- Brink I, Hoegerle S, Klisch J, Bley TA. Imaging of pheochromocytoma and paraganglioma. Fam Cancer 2005;4:61-8. [Crossref] [PubMed]
- Strajina V, Dy BM, Farley DR, Richards ML, McKenzie TJ, Bible KC, Que FG, Nagorney DM, Young WF, Thompson GB. Surgical Treatment of Malignant Pheochromocytoma and Paraganglioma: Retrospective Case Series. Ann Surg Oncol 2017;24:1546-50. [Crossref] [PubMed]
- Jimenez C, Waguespack SG. Functional imaging for pheochromocytoma-paraganglioma: a step closer to understanding its place in clinical practice. Endocrine 2015;50:6-8. [Crossref] [PubMed]
- Brito JP, Asi N, Gionfriddo MR, Norman C, Leppin AL, Zeballos-Palacios C, Undavalli C, Wang Z, Domecq JP, Prustsky G, Elraiyah TA, Prokop LJ, Montori VM, Murad MH. The incremental benefit of functional imaging in pheochromocytoma/paraganglioma: a systematic review. Endocrine 2015;50:176-86. [Crossref] [PubMed]
- Maurea S, Cuocolo A, Imbriaco M, Pellegrino T, Fusari M, Cuocolo R, Liuzzi R, Salvatore M. Imaging characterization of benign and malignant pheochromocytoma or paraganglioma: comparison between MIBG uptake and MR signal intensity ratio. Ann Nucl Med 2012;26:670-5. [Crossref] [PubMed]
- Furcea L, Mois E, Al Hajjar N, Seicean A, Badea R, Graur F. Pancreatic Gangliocytic Paraganglioma- CEUS Appearance. J Gastrointestin Liver Dis 2017;26:336. [PubMed]
- Friedrich-Rust M, Glasemann T, Polta A, Eichler K, Holzer K, Kriener S, Herrmann E, Nierhoff J, Bon D, Bechstein WO, Vogl T, Zeuzem S, Bojunga J. Differentiation between benign and malignant adrenal mass using contrast-enhanced ultrasound. Ultraschall Med 2011;32:460-71. [Crossref] [PubMed]
- Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic Tests and Biomarkers for Pheochromocytoma and Extra-adrenal Paraganglioma: From Routine Laboratory Methods to Disease Stratification. Endocr Pathol 2012;23:4-14. [Crossref] [PubMed]
- Martucci VL, Pacak K. Pheochromocytoma and Paraganglioma: Diagnosis, Genetics, Management, and Treatment. Curr Probl Cancer 2014;38:7-41. [Crossref] [PubMed]
- King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine 2010;37:403-7. [Crossref] [PubMed]


