
Potential value of apparent diffusion coefficient in the evaluation of hepatic pseudolymphoma
Introduction
Hepatic pseudolymphoma, also called reactive lymphoid hyperplasia or nodular lymphoid lesion, is a rare benign non-neoplastic lesion that is histopathologically composed of lymphoid follicles, densely-infiltrated lymphoplasmacytic cells and epithelioid cells (1). The incidence of this disease among females is nine times that of males, which shows a significant gender preference (2). Most hepatic pseudolymphomas are asymptomatic, with an average onset age of 50 years (2).
Due to the confusing imaging features of hepatic pseudolymphoma with hepatocellular carcinoma (HCC) or hepatic metastasis (3), the final pathological diagnosis of hepatic pseudolymphoma in clinic depends on surgical resection. Given the benignity of hepatic pseudolymphoma, the possibility of obtaining pathological tissues by a more minimally invasive approach should be explored so as to avoid unnecessary surgical management.
Herein, we reported a case of hepatic pseudolymphoma confirmed by ultrasound-guided biopsy and fully illustrated its performance by ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT. Finally, the imaging differential diagnosis of hepatic pseudolymphoma was analyzed so as to minimize misdiagnosis and inappropriate clinical management.
Case presentation
A 50-year-old asymptomatic female was found with a hepatic lesion during a routine health check and was thus admitted to our institution for further assessment. Laboratory examination indicated the liver function was in the normal range. The elevated hepatitis B virus indicator showed an infection with hepatitis B virus, but the patient had been regularly taking antiviral medication. She had undergone two operations, including subtotal thyroidectomy 7 years ago (a benign lesion) and cholecystectomy due to gallstones ten years ago. The patient had no other chronic diseases, such as hypertension and diabetes, and had no harmful health habits, such as smoking or drinking. Clinical/imaging evaluation of the liver showed normal liver morphology with smooth margin and homogeneous texture, balanced ratio of hepatic lobe to hepatic dysmorphia, and no obvious signs of cirrhosis or portal hypertension.
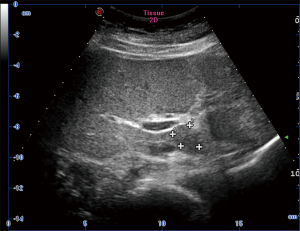
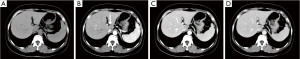
Hepatic ultrasonography showed a hypoechoic nodule in segment 1 with clear boundary (Figure 1). Non-enhanced CT indicated a homogeneously hypoattenuating lesion (45.7 HU, 1.7×2.5 cm2) in segment I. Hepatic contrast-enhanced CT revealed a hypoattenuating nodule (61.9 HU) with minimal perinodular enhancement during the arterial phase. The lesion was also hypoattenuating during the portal and delayed phases (79.8 and 81.5 HU, respectively; Figure 2).
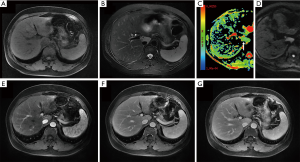
Conventional MRI of the hepatic lesion showed hypointense signals on T1-weighted images (T1WI) and hyperintense signals on T2-weighted images (T2WI) with clear boundaries. In the out phase, the signals of the lesion were without any deficiency. The diffusion weighted imaging (DWI) sequence of the lesion showed hyperintense signals with a low apparent diffusion coefficient (ADC) (0.95×10−3 mm2/s) at b=0, 1,000 mm2/s, which indicated the diffusion restriction of water molecules. Contrast-enhanced MRI showed a heterogeneous enhancement of the lesion during the arterial phase and mildly hyperintense signals during the portal and delayed phases (Figure 3), which suggested a prolonged enhancement pattern. Moreover, no enlarged lymph node was found in the hilar and retroperitoneal region.
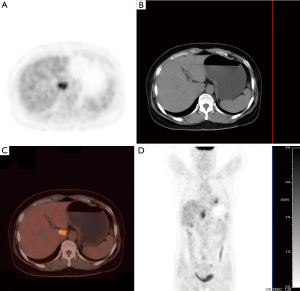
The hepatic lesion was further evaluated by non-enhanced PET/CT scan (Siemens Medical Solutions USA, Inc., Malvern, PA, USA), which showed an irregular hypoattenuating lesion with ill-defined boundary and an increased radioactive uptake (SUVmax 6.4) (Figure 4). No abnormal (18F)s-fluorodeoxyglucose (FDG) metabolism was found in other parts of the body.
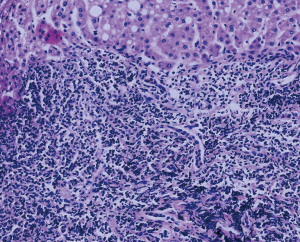
An ultrasound-guided needle biopsy was further performed for diagnosis. Pathological tissues were successfully obtained. The pathological results revealed the infiltration of numerous B lymphocytes and the involvement of T lymphocytes into the hepatic cells, but no hepatocyte lobular structure (Figure 5). Immunohistochemistry showed hepatocyte (+), CK19 (+), CD20 (+), CD3 (+), AFP (−), CD34 (−), GPC-3 (−), TDT (−), CD1a (−), and Ki-67 (low). The pathological diagnosis suggested hepatic pseudolymphoma and low-grade B-cell lymphoma, and was then confirmed by a clonal gene rearrangement test, which showed “negative”. Finally, the morphology, immunophenotype, and clonal gene rearrangement test results together confirmed the diagnosis of hepatic pseudolymphoma. During 23 months of follow-up, the patient did not complain about any apparent discomfort. The patient underwent imaging examinations several times, including recent hepatic ultrasound, which indicated no significant change in lesion size.
Discussion
Pseudolymphoma is a proliferative disorder of lymphoid tissues and can attack various parts of the body, including the intestine, lungs, skin, eye orbits, thyroid and less commonly, the liver (2). Since the first report of hepatic pseudolymphoma in 1981 (4), about 50 cases of sporadic hepatic pseudolymphomas have been reported (5). Pathologically, the differential diagnosis of hepatic pseudolymphoma from low-grade B-cell lymphoma is often challenging and should be assisted by gene rearrangements and in situ hybridization (6). In the present case, however, the histopathology of tissues obtained by biopsy failed to distinguish these two lymphomas. Nevertheless, a definitive diagnosis was finally confirmed by clonal gene rearrangement test.
The etiology of hepatic pseudolymphoma remains unknown, but a significant proportion of these patients are combined with hepatic diseases, including hepatitis virus infection, primary biliary cirrhosis, and non-alcoholic steatohepatitis (2,5). Patients with hepatic pseudolymphoma are prone to autoimmune diseases, including autoimmune thyroiditis, Sjogren’s disease, antiphospholipid syndrome, CREST syndrome, and Takayasu disease (2). Interestingly, the present patient suffered from chronic hepatitis, but not autoimmune disease. In brief, the specific molecular mechanism of hepatic pseudolymphoma may be critically related to hepatitis virus infection, and intrahepatic and external autoimmune processes, but still requires further exploration.
Hepatic pseudolymphoma, usually in diameter of less than 2 cm, is often solitary and occasionally in group (5). Ultrasonography indicated a hypoechoic lesion had surrounding vascular signals (1,2,7). Contrast-enhanced ultrasonography showed a wash-out enhancement pattern of the lesion, which may be misdiagnosed as HCC (7). Non-enhanced CT of lesions showed hypodensity. The abundant vascularity, ring enhancement and vessel-penetrating sign on contrast-enhanced CT were considered to be diagnostic clues (8,9). Conventional MRI showed the lesion was round with homogeneously hypointense signals on T1WI and hyperintense signals on T2WI (5,7,10,11). The DWI sequence of the tumor showed hyperintense signals with significantly low ADC (5,7,10,11). Contrast-enhanced MRI revealed the most common pattern was ring enhancement, the pathological basis of which was lymphocytic infiltration around the nodule (5,7,10). Moreover, pseudocapsules were reported (5).
Because more than one-quarter of hepatic pseudolymphomas are associated with tumors (mainly gastric cancer and colon cancer), ring-enhanced hepatic pseudolymphomas could be easily misdiagnosed as metastatic tumors. In our case, the lesion showed a prolonged enhancement on contrast-enhanced MRI. As reported, lesions during Gd-EOB-DTPA or Eovist-enhanced hepatobiliary phases showed hypointense signals (2,7,9,11) or slightly hypointense signals due to less uptake of the contrast agent (8). Therefore, liver-specific contrast agents may limit the diagnostic value for hepatic pseudolymphoma. The intense uptake of hepatic pseudolymphomas on non-enhanced PET/CT may lead to misdiagnosis of hepatic malignancy (12). In our case, despite the intense FDG uptake, the hepatic pseudolymphoma was still at a lower metabolism compared with the well-differentiated HCC (13).
We emphasize the diagnostic value of ADC in differentiating hepatic pseudolymphoma from hepatic neoplastic lesions. Studies demonstrate hepatic pseudolymphoma should be differentiated from HCC and hepatic metastases (5,11). A study of hepatic pseudolymphoma confirmed that the diffuse infiltration of small lymphocytes resulted in a significantly limited diffusion of water molecules with an ADC of (0.82±0.45)×10−3 mm2/s (b=0, 500 mm2/s) (5). Although the b value differed, ADC in our case still fell in the scope of the above research. Moreover, lymphoid tissue-derived hepatic lymphomas with low ADC (0.83×10−3 mm2/s) can be significantly differentiated from benign disorders (1.40×10−3 mm2/s), such as hepatic focal nodular hyperplasia and adenomas, and from malignant tumors (1.06×10−3 mm2/s), such as hepatocellular cancer, cholangiocarcinoma, and metastatic tumors (14). Therefore, low ADC may be an important clue in the diagnosis of hepatic pseudolymphoma.
However, ADC is unable to distinguish between hepatic pseudolymphoma and hepatic lymphoma because ADC is low in both cases due to the monoclonal or polyclonal dense lymphocytes. We believe these two lymphomas may be feasibly differentiated by size and enhancement pattern. Hepatic pseudolymphomas are generally smaller (less than 2 cm in diameter) than hepatic lymphomas, which often lump together. Also on contrast-enhanced CT and MRI, hepatic pseudolymphomas usually show marginal enhancement, compared with the less marginal enhancement of hepatic lymphomas. In addition, clinical characteristics also may help with the differentiation, since hepatic pseudolymphomas are generally asymptomatic without abnormal laboratory indicators, whereas hepatic lymphomas are symptomatic with abnormal laboratory indicators.
At present, the clinical application of quantified ADC is limited by the difficulty of re-emergence. On the one hand, the acquisition of ADC is affected by many factors, including hardware devices (e.g., 1.5 and 3 T field strength), scan sequence parameters (e.g., b-values) and biological factors (e.g., benign and malignant liver diseases). On the other hand, different ADC thresholds are used in different studies for the differential diagnosis of benign and malignant liver lesions (15). These factors render the absence of ADC in the 2017 Version of LI-RADS (16). Nevertheless, DWI sequences including ADC are useful complements to conventional MRI sequences in clinical evaluation of hepatic tumors (17).
Hepatic pseudolymphoma is usually pathologically diagnosed by surgical resection, which also helps to achieve therapeutic goals (5,6). In a few cases, pathological tissues were obtained by liver biopsy for the final diagnosis (1,6). In the follow-up cases, the diameter of hepatic pseudolymphomas generally decreased. Since hepatic pseudolymphoma is free from malignant transformation (3), unnecessary surgical treatment should be avoided. In the present case, due to the possibility of benignity, pathological tissues were obtained through biopsy for a final diagnosis, which helped the patient avoid surgery. Therefore, we suggest biopsy as a diagnostic procedure and encourage follow-up for such tumors so as to avoid unnecessary surgical resection.
Generally, the imaging characteristics of hepatic pseudolymphoma are diverse, but the ADC of DWI sequence may help to narrow the differential diagnosis range. The size and enhancement pattern of the lesions may contribute to the differentiation between hepatic pseudolymphoma and hepatic lymphoma. A biopsy is the appropriate way to obtain a final diagnosis of hepatic pseudolymphoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Calvo J, Carbonell N, Scatton O, Marzac C, Ganne-Carrie N, Wendum D. Hepatic nodular lymphoid lesion with increased IgG4-positive plasma cells associated with primary biliary cirrhosis: a report of two cases. Virchows Arch 2015;467:613-7. [Crossref] [PubMed]
- Taguchi K, Kuroda S, Kobayashi T, Tashiro H, Ishiyama K, Ide K, Ohira M, Tahara H, Arihiro K, Ohdan H. Pseudolymphoma of the liver: a case report and literature review. Surg Case Rep 2015;1:107. [Crossref] [PubMed]
- Moon WS, Choi KH. Reactive lymphoid hyperplasia of the liver. Clin Mol Hepatol 2013;19:87-91. [Crossref] [PubMed]
- Snover DC, Filipovich AH, Dehner LP, Krivit W. ‘Pseudolymphoma’. A case associated with primary immunodeficiency disease and polyglandular failure syndrome. Arch Pathol Lab Med 1981;105:46-9. [PubMed]
- Zhou Y, Wang X, Xu C, Zhou G, Zeng M, Xu P. Hepatic pseudolymphoma: imaging features on dynamic contrast-enhanced MRI and diffusion-weighted imaging. Abdom Radiol (NY) 2018;43:2288-94. [Crossref] [PubMed]
- Zen Y, Fujii T, Nakanuma Y. Hepatic pseudolymphoma: a clinicopathological study of five cases and review of the literature. Mod Pathol 2010;23:244-50. [Crossref] [PubMed]
- Kunimoto H, Morihara D, Nakane SI, Tanaka T, Yokoyama K, Anan A, Takeyama Y, Irie M, Shakado S, Noritomi T, Takeshita M, Yoshimitsu K, Sakisaka S. Hepatic Pseudolymphoma with an Occult Hepatitis B Virus Infection. Intern Med 2018;57:223-30. [Crossref] [PubMed]
- Osame A, Fujimitsu R, Ida M, Majima S, Takeshita M, Yoshimitsu K. Multinodular pseudolymphoma of the liver: computed tomography and magnetic resonance imaging findings. Jpn J Radiol 2011;29:524-7. [Crossref] [PubMed]
- Yoshida K, Kobayashi S, Matsui O, Gabata T, Sanada J, Koda W, Minami T, Ryu Y, Kozaka K, Kitao A. Hepatic pseudolymphoma: imaging-pathologic correlation with special reference to hemodynamic analysis. Abdom Imaging 2013;38:1277-85. [Crossref] [PubMed]
- Sonomura T, Anami S, Takeuchi T, Nakai M, Sahara S, Tanihata H, Sakamoto K, Sato M. Reactive lymphoid hyperplasia of the liver: Perinodular enhancement on contrast-enhanced computed tomography and magnetic resonance imaging. World J Gastroenterol 2015;21:6759-63. [Crossref] [PubMed]
- Kwon YK, Jha RC, Etesami K, Fishbein TM, Ozdemirli M, Desai CS. Pseudolymphoma (reactive lymphoid hyperplasia) of the liver: A clinical challenge. World J Hepatol 2015;7:2696-702. [Crossref] [PubMed]
- Zhong X, Dong A, Dong H, Wang Y. FDG PET/CT in 2 Cases of Hepatic Pseudolymphoma. Clin Nucl Med 2018;43:e166-9. [PubMed]
- Bertagna F, Bertoli M, Bosio G, Biasiotto G, Sadeghi R, Giubbini R, Treglia G. Diagnostic role of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int 2014;8:493-500. [Crossref] [PubMed]
- Colagrande S, Calistri L, Grazzini G, Nardi C, Busoni S, Morana G, Grazioli L. MRI features of primary hepatic lymphoma. Abdom Radiol (NY) 2018;43:2277-87. [Crossref] [PubMed]
- Shenoy-Bhangle A, Baliyan V, Kordbacheh H, Guimaraes AR, Kambadakone A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J Hepatol 2017;9:1081-91. [Crossref] [PubMed]
- Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, Chernyak V, Bashir MR, Kono Y, Do RK, Mitchell DG, Kamaya A, Hecht EM, Sirlin CB. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics 2017;37:1994-2017. [Crossref] [PubMed]
- Chilla GS, Tan CH, Xu C, Poh CL. Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant Imaging Med Surg 2015;5:407-22. [PubMed]


