
Circumferential strain rate to detect lipopolysaccharide-induced cardiac dysfunction: a speckle tracking echocardiography study
Introduction
Septic cardiomyopathy (SC), a complication of severe sepsis and septic shock, remains a clinical challenge (1). As a major outcome predictor, SC occurs to more than 40% of septic patients and leads to 70% of sepsis-related deaths (2). Decades of research on SC have not yet uncovered the mechanism behind SC.
Lipopolysaccharide (LPS) is a major component in the outer membrane of gram-negative bacteria and serves as a key stimulator of sepsis (3). It can evoke inflammatory responses in both innate immune cells and non-immune cells (e.g., cardiomyocytes) (4). Balija et al. found 6 hours of LPS administration not only strengthened negative inotropic effects, but also altered volume loading conditions in septic cardiac abnormalities (5). Cardiac dysfunction frequently occurs in patients with sepsis and animals injected with LPS (6,7). At present, LPS-induced myocardial dysfunction models are widely used to study SC.
Echocardiography, a real-time, non-invasive and cost-effective technology, is being widely replicated worldwide (8). However, its reference values, including fractional shortening (FS) or ejection fraction (EF), are calculated based on normal systemic vascular resistance (9). These values may underestimate the severity of SC patients with severe sepsis and vasodilation. Furthermore, it may even mask cardiac impairment due to severe reduction of afterload in septic shock (10).
Speckle tracking echocardiography (STE), a novel echocardiographic technique, has shown its power to provide information of higher sensitivity and specificity by capturing segmental tissue motion in multiple planes and axes (11,12). Strain analysis focuses on regional and global left ventricular (LV) function over the cardiac cycle and can precisely evaluate myocardial performance (13). Although STE can detect the abnormal LV strain and torsion in SC (14), strain rate imaging (SRI), especially circumferential strain rate (SRcirc), is rarely used (15).
In the present study, we investigated the efficiency of SRcirc in detecting LPS-induced cardiac dysfunction in mice.
Methods
Animals and ethics
All procedures were approved by the Live Animals in Teaching and Research Committee (Approval ID: IACUC-1703039) and relevant regulations set by Nanjing Medical University. All experiments were performed according to the “principles and guide for the care and use of laboratory animals” published by the National Institutes of Health (No. 85-23, revised 1996).
C57BL/6J mice (aged 8–10 weeks, weighing 23–26 g) were obtained from the Model Animal Research Center of Nanjing University. The mice were kept in a 12 h/12 h-light/dark cycle at 21–23 °C for at least 10 days and fed with a standard chow diet before experiments. Baseline indexes (body weight, temperature and heart rate) were recorded.
Study protocol
A total of 30 mice were randomly assigned to saline (n=10), LPS 10 mg/kg (n=10) and LPS 20 mg/kg (n=10) groups. Additionally, to detect circumferential changes, we assessed cardiac function at baseline, 6 h and 20 h after injection. After that, strain analysis was performed by STE. Standard M-mode tracing parameters were tested and STE conducted to obtain SRcirc values at 6 h after injection.
LPS injection
First, E. coli LPS (O111:B4, Sigma, USA) was dissolved in sterile physiological saline (1 mg/mL) (16). Then, the mice were injected intraperitoneally with 10 or 20 mg/kg LPS or the same volume of saline, respectively.
M-mode echocardiography
The mice were anesthetized (1% isoflurane) and imaged under light sedation at room temperature by an experienced operator. Echocardiography was performed with a Vivid 7 ultrasound (GE, Wisconsin, USA) equipped with an il3L intraoperative linear probe at 10.0–14.0 MHz. M-mode images were obtained at the mid-papillary level in parasternal short-axis views. Parameters included LV internal diameter at diastole (LVIDd), LV internal diameter at systole (LVIDs), LV volume at diastole (LVVd), LV volume at systole (LVVs), EF, and FS.
STE
Using Echopac PC software (version 113.1, GE, Horten, Norway), we performed STE offline to process SRcirc. The first STI procedure was manually contouring the area of interest between endocardial and epicardial borders. Subsequently, grayscale images were analyzed using speckle tracking software following frame-to-frame movement of stable patterns of natural acoustic markers (or speckles). Finally, global SRcirc at peak systole (SRs), global SRcirc at peak early diastole (SRe) and global SRcirc at peak late diastole (SRa) were calculated (17). The data were analyzed by two independent investigators.
Biochemical analysis
Serum cardiac troponin-T (cTnT) was determined using a cardiac troponin T quantitative assay from Roche Diagnostics GmbH (Mannheim, Germany). Creatine kinase-MB (CK-MB) was determined on a VITROS-5600 automated biochemical analyzer from Ortho-clinical Diagnostics (New York, USA).
Statistical analysis
All experiments were repeated to obtain with repetitions qualitatively similar data. All data was analyzed by SPSS 17.0 (Chicago, USA) and GraphPad Prism 6.0 (CA, USA) software. The between-group difference was analyzed with unpaired Student t-test or one-way ANOVA test. Values were expressed as mean ± standard deviation, and P<0.05 was considered statistically significant.
Results
Basic information of study animals
The mice were randomly assigned according to study protocols. Only males were used to exclude possible confounders caused by hormonal fluctuation. There was no statistically significant difference in baseline body weight, temperature and heart rate between saline, 10 mg/kg and 20 mg/kg groups (P>0.05) (Table 1).
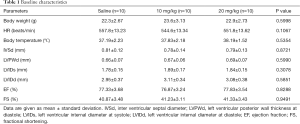
Full table
Effects of different doses of LPS in altering cardiac function
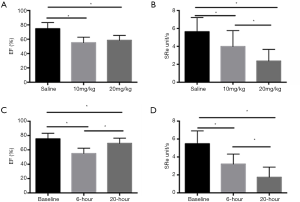
To distinguish the effect of LPS on cardiac dysfunction, mice were treated with low or high dose of LPS. At 6 h after injection, the systolic volume (LVIDs, LVVs) increased and the overall LV function decreased significantly in two groups: EF (saline vs. 10 mg/kg, 74.57%±5.12% to 55.36%±7.42%; saline vs. 20 mg/kg, 74.57%±5.12% to 58.31%±11.68%; P<0.05) and FS (saline vs. 10 mg/kg, 42.56%±3.17% to 25.43%±4.32%; saline vs. 20 mg/kg, 42.56%±3.17% to 29.67%±8.79%; P<0.05). Additionally, no differences of myocardial volume (LVIDs, 2.39±0.33 to 2.11±0.42 mm; LVVs, 37.67±15.67 to 33.41±8.37 mm; P>0.05) and load-dependent indexes (EF, 55.36%±7.42% to 58.31%±11.68%; FS, 25.43%±4.32% to 29.67%±8.79%; P>0.05) were found between the low and high dose groups (Table 2).
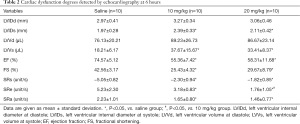
Full table
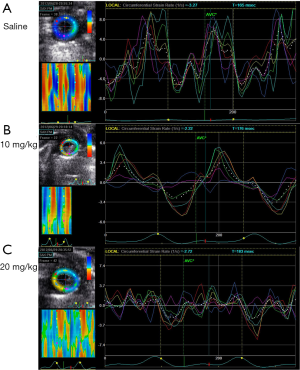
Compared with M-mode echo, STI detected the remarkable decline of SRcirc after LPS injection. The 20 mg/kg group showed a larger decrease in SRcirc (Figures 1,2), including SRs (saline vs. 10 mg/kg, −5.05±0.82 to −2.30±0.94 unit/s; saline vs. 20 mg/kg, −5.05±0.82 to −1.82±0.85 unit/s; P<0.05), SRe (baseline vs. 10 mg/kg, 5.23±2.30 to 3.18±0.83 unit/s; baseline vs. 20 mg/kg, 5.23±2.30 to 1.76±1.05 unit/s; P<0.05) and SRa (baseline vs. 10 mg/kg, 2.23±1.01 to 1.65±0.80 unit/s; baseline vs. 20 mg/kg, 2.23±1.01 to 1.46±0.77 unit/s; P<0.05) (Table 2). Additionally, SRe elevated between 10 and 20 mg/kg dose group (3.18±0.83 vs. 1.76±1.05 unit/s; P<0.05). The results demonstrated that STI rather than M-mode could detect the LPS-dose-dependent change.
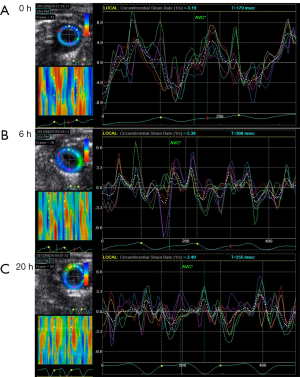
Changes of cardiac function at different time points following LPS challenge
To investigate LPS-induced circumferential change, M-mode echo and STI were used after the injection of 10 mg/kg LPS. After the mice took 10 mg/kg LPS, their systolic volumes (LVIDs, LVVs) were elevated and their overall LV function declined significantly at both time points: EF (baseline vs. 6-hour, 76.87%±5.24% to 55.36%±7.42%; baseline vs. 20-hour, 76.87%±5.24% to 71.31%±11.68%; P<0.05) and FS (baseline vs. 6-hour, 41.23%±3.11% to 25.43%±4.32%; baseline vs. 20-hour, 41.23%±3.11% to 35.67%±8.79%; P<0.05). Moreover, compared with those at 6 h, the systolic myocardial volume declined while the EF and FS elevated at 20 h. LVID (1.97±0.43 vs. 2.39±0.33 mm, P<0.05), LVV (23.41±8.37 vs. 37.67±15.67 µL, P<0.05), EF (71.31%±11.68% vs. 55.36%±7.42%, P<0.05) and FS (35.67%±8.79% vs. 25.43%±4.32%, P<0.05) were observed (Table 3).
Full table
In contrast, strain rate analysis also detected the declining LV myocardial function after 10 mg/kg LPS administration (Figures 2,3). SRcirc significantly declined from baseline to 6 or 20 h after the treatment, including SRs (baseline vs. 6-hour, −5.05±0.82 to −2.30±0.94 unit/s; baseline vs. 20-hour, −5.05±0.82 to −2.19±0.56 unit/s; P<0.05), SRe (baseline vs. 6-hour, 5.23±2.30 to 3.18±0.83 unit/s; baseline vs. 20-hour, 5.23±2.30 to 1.57±0.75 unit/s; P<0.05) and SRa (baseline vs. 6-hour, 2.23±1.01 to 1.65±0.80 unit/s; baseline vs. 20-hour, 2.23±1.01 to 1.84±0.85 unit/s; P<0.05) (Table 3). In addition, SRcirc significantly decreased from 6 to 20 h (SRe, 3.18±0.83 to 1.57±0.75 unit/s; P<0.05). The results showed SRcirc was more sensitive than conventional M-mode echo to identify time-dependent circumferential change after LPS injection.
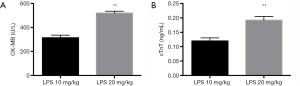
Serum evidences indicated LPS administration worsened SC
In the serum, the expressions of CK-MB and cTnT indicate myocardial damage (17). With biochemical analysis, the serum CK-MB (519.60±7.23 vs. 314.00±10.05 U/L) and cTnT (0.19±0.01 vs. 0.12±0.01 ng/mL) levels elevated in the high-dose LPS group (Figure 4). The result revealed the severer myocardial damage and inflammation associated with LPS administration.
Discussion
In this study, SRI showed favorable efficiency in evaluating LPS-induced myocardial dysfunction in mice. At 6 h after injection, M-mode failed to detect the cardiac impairment while SRcirc found myocardial function degraded in the high dose group. In 10 mg/kg group, compared with those at 6 h, M-mode echo parameters increased but SRcirc values decreased at 20 h after LPS injection. Figures 1 and 3 showed the dose- and time-dependent effect of LPS detected by SRcirc values (especially SRe) in SC. Finally, we found that SRcirc values increased with CK-MB and cTnT levels, indicating the ability of LPS to worsen SC.
Myocardial dysfunction often raises in SC but its underlying physiopathology remains unclear. Studies have revealed that mortality is caused by septic shock, reduced cardiac output, and elevated systemic vascular resistance in SC (18,19). Echocardiography is most preferred to detect the myocardial dysfunction during SC (20). However, our data showed the systolic myocardial volumes declined and the load-dependent indexes elevated by M-mode echo at 20 h, compared with those at 6 h. It suggested that M-mode tracing failed to detect cardiac dysfunction (10) due to unstable hemodynamic conditions in SC. Moreover, M-mode echo may be not powerful enough to assess myocardial performance in SC (21), because of decreased myocardial contractility and changed cardiac loading conditions (22-25). Conversely, significant decline in SRe was detected by STE, suggesting that STE is effective to detect the impaired LV strain and torsion changes in septic patients (11,14,26). But using comprehensive SRI to assess SC has not been experimented.
In this study, we identified LPS-induced cardiac dysfunction with conventional echocardiography and SRI (SRs, SRe and SRa). As a result, no differences in LVID, LVV at systole, EF and FS were observed in the high-dose group by M-mode tracing. Conversely, STI detected the remarkable decline in SRcirc, suggesting its higher sensitivity and reliability in evaluating cardiac inotropism in unstable hemodynamic conditions than conventional echocardiography. More work is needed to determine the cutoff values of SRcirc to diagnose SC. The efficiency of SRcirc combined with conventional echocardiography should also be evaluated.
Interestingly, a significant difference was observed in SRe, which was time-dependent and dose-dependent. Circumferential strain was used to detect LPS-induced cardiac dysfunction and showed favorable accuracy and reproducibility in our previous study (24,25). Animal experiments have demonstrated the peak systolic strain rate is weakly influenced by afterload and may be a better surrogate parameter than strain to test intrinsic contractility (27). However, few studies have reported the usefulness of diastolic strain rate in evaluating intrinsic contractility. James et al. explored the role of strain rate, including diastolic strain rate, in the assessment of left myocardial function in preterm infants using tissue Doppler imaging (28). Here we found that the strain rate could be used to assess LV function in unstable hemodynamic conditions. Among various SRI parameters, SRe is more sensitive and reliable in evaluating cardiac contractility in unstable afterload.
Additionally, serum markers (CK-MB and cTnT) were used to quantify the myocardial injury and the myocardial damage by sepsis in a noninvasive and available way. We found the high levels of CK-MB and cTnT caused by augmented myocardial injury. Serum analysis confirmed the ongoing myocardial injury within 20 h after LPS injection. Consequently, conventional echocardiography only found the increased load-dependent indexes (EF and FS) after the 20th h, failing to detect the cardiac dysfunction from LPS-induced myocardial injury. The serum analysis results confirmed the dose-dependent LPS effect illustrated by SRcirc, advocating that SRcirc has a high diagnostic value for cardiac dysfunction in severe sepsis.
Our previous study demonstrated LPS administration caused myocardial injury and inflammation, indicating significant reduction of afterload condition. This study verified SRcirc had significant diagnostic value under unstable hemodynamic conditions in SC. Future clinical trials are needed to establish a standard evaluation system based on STI for evaluating SC.
Limitations
Limitations are as follows: (I) more parameters obtained by SRI are needed to support the accuracy and reproducibility of SRI in severe sepsis, such as radial strain rate; (II) strain rate may show species-associated difference between the murine and the human; large animal models using pig or canine are needed to enhance our finding. Therefore, the combination of conventional echo and SRI may be more feasible in assessing cardiac function in patients with severe sepsis.
Conclusions
SRcirc is sensitive to detect LPS-induced cardiac dysfunction. More work should be done to test the efficiency of combined STE and conventional echocardiography for diagnosing human SC.
Acknowledgements
Funding: This study was supported by Jiangsu Provincial Key Discipline of Medicine (ZDXKA2016003), by the Priority Academic Program Development of Jiangsu higher Education Institutions (PAPD), by Postgraduate Research & Practice Innovation Program of Jiangsu Province, by Postgraduate international exchanges and cooperation project of Nanjing Medical University, by China Scholarship Council, by Natural Science Foundation of Jiangsu Province (Grant No. BK20161057) and National Natural Science Foundation of China (Grant No. 81301616, 81601516 and 81271589).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures were approved by the Live Animals in Teaching and Research Committee (Approval ID: IACUC-1703039) and relevant regulations set by Nanjing Medical University.
References
- Ehrman RR, Sullivan AN, Favot MJ, Sherwin RL, Reynolds CA, Abidov A, Levy PD. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care 2018;22:112-20. [Crossref] [PubMed]
- Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A, Carriedo D, Valledor M, De Frutos M, López MJ, Caballero A, Guerra J, Alvarez B, Mayo A, Villar J. Grupo de Estudios y Análisis en Cuidados Intensivos. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008;12:R158. [Crossref] [PubMed]
- Kong W, Kang K, Gao Y, Liu H, Meng X, Yang S, Yu K, Zhao M. Dexmedetomidine alleviates LPS-induced septic cardiomyopathy via the cholinergic anti-inflammatory pathway in mice. Am J Transl Res 2017;9:5040-7. [PubMed]
- Uchida K, Urata H, Mohri Y, Inoue M, Miki C, Kusunoki M. Seprafilm Does Not Aggravate Intraperitoneal Septic Conditions or Evoke Systemic Inflammatory Response. Surg Today 2005;35:1054-9. [Crossref] [PubMed]
- Balija TM, Lowry SF. Lipopolysaccharide and sepsis-associated myocardial dysfunction. Curr Opin Infect Dis 2011;24:248-53. [Crossref] [PubMed]
- Yang P, Han Y, Gui L, Sun J, Chen YL, Song R, Guo JZ, Xie YN, Lu D, Sun L. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-κB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochem Pharmacol 2013;85:1124-33. [Crossref] [PubMed]
- Yücel G, Zhao Z, El-Battrawy I, Lan H, Lang S, Li X, Buljubasic F, Zimmermann WH, Cyganek L, Utikal J, Ravens U, Wieland T, Borggrefe M, Zhou XB, Akin I. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci Rep 2017;7:2935-41. [Crossref] [PubMed]
- Omoto R, Yokote Y, Takamoto S, Kyo S, Ueda K, Asano H, Namekawa K, Kasai C, Kondo Y, Koyano A. The development of real-time two-dimensional Doppler echocardiography and its clinical significance in acquired valvular diseases. With special reference to the evaluation of valvular regurgitation. Jpn Heart J 1984;25:325-40. [Crossref] [PubMed]
- Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol 2012;17:5-11. [PubMed]
- Werdan K, Oelke A, Hettwer S, Nuding S, Bubel S, Hoke R, Russ M, Lautenschläger C, Mueller-Werdan U, Ebelt H. Septic cardiomyopathy: hemodynamic quantification, occurrence, and prognostic implications. Clin Res Cardiol 2011;100:661-8. [Crossref] [PubMed]
- Chrzanowski L, Uznańska B, Plewka M, Krzemińska-Pakuła M, Kasprzak J. M-mode speckle tracking--a novel echocardiographic approach to assess left ventricular torsional deformation. Kardiol Pol 2009;67:1070-76. [PubMed]
- D'Ascenzi F, Caselli S, Solari M, Pelliccia A, Cameli M, Focardi M, Padeletti M, Corrado D, Bonifazi M, Mondillo S. Novel echocardiographic techniques for the evaluation of athletes' heart: A focus on speckle-tracking echocardiography. Eur J Prev Cardiol 2016;23:437-46. [Crossref] [PubMed]
- Kalay N, Celik A, Inanc T, Dogan A, Ozdogru I, Kaya MG, Oguzhan A, Topsakal R, Ergin A. Left Ventricular Strain and Strain Rate Echocardiography Analysis in Patients with Total and Subtotal Occlusion in the Infarct-Related Left Anterior Descending Artery. Echocardiography 2011;28:203-9. [Crossref] [PubMed]
- Bloechlinger S, Berger D, Bryner J, Wiegand J, Dünser MW, Takala J. Left ventricular torsion abnormalities in septic shock and corrective effect of volume loading: a pilot study. Can J Cardiol 2013;29:1665-71. [Crossref] [PubMed]
- Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, La Gerche A, Rademakers F, Herijgers P, D'hooge J. Assessment of strain and strain rate by two-dimensional speckle tracking in mice: comparison with tissue Doppler echocardiography and conductance catheter measurements. Eur Heart J Cardiovasc Imaging 2013;14:765-73. [Crossref] [PubMed]
- Heimdal A, StÃylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013-9. [Crossref] [PubMed]
- Niu J, Azfer A, Kolattukudy PE. Protection against lipopolysaccharide-induced myocardial dysfunction in mice by cardiac-specific expression of soluble Fas. J Mol Cell Cardiol 2008;44:160-9. [Crossref] [PubMed]
- Fink MP. Animal models of sepsis. Virulence 2014;5:143-53. [Crossref] [PubMed]
- Wiel E, Costecalde ME, Lebuffe G, Corseaux D, Jude B, Bordet R, Tavernier B, Vallet B. Activated protein C increases sensitivity to vasoconstriction in rabbit Escherichia coli endotoxin-induced shock. Crit Care 2006;10:R47. [Crossref] [PubMed]
- Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care 2011;1:6. [Crossref] [PubMed]
- Peters M J, Brierley J. No representation without taxation: declaration of (load) independence in septic cardiomyopathy. Pediatr Crit Care Med 2012;13:349-50. [Crossref] [PubMed]
- Price S, Anning PB, Mitchell JA, Evans TW. Myocardial dysfunction in sepsis: mechanisms and therapeutic implications. Eur Heart J 1999;20:715-24. [Crossref] [PubMed]
- Belcher E, Mitchell J, Evans T. Myocardial dysfunction in sepsis: no role for NO? Heart 2002;87:507-9. [Crossref] [PubMed]
- Chu M, Gao Y, Zhang Y, Zhou B, Wu B, Yao J, Xu D. The role of speckle tracking echocardiography in assessment of lipopolysaccharide-induced myocardial dysfunction in mice. J Thorac Dis 2015;7:2253-61. [PubMed]
- Chu M, Gao Y, Zhou B, Wu B, Wang J, Xu D. Circumferential Strain Can Be Used to Detect Lipopolysaccharide-Induced Myocardial Dysfunction and Predict the Mortality of Severe Sepsis in Mice. Plos One 2016;11:e0155346. [Crossref] [PubMed]
- Basu S, Frank LH, Fenton KE, Sable CA, Levy RJ, Berger JT. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med 2012;13:259-64. [Crossref] [PubMed]
- Mirea O, Duchenne J, Voigt JU. Recent advances in echocardiography: strain and strain rate imaging. F1000Res 2016;5:787-96. [Crossref] [PubMed]
- James AT, Corcoran JD, Breatnach CR, Franklin O, Mertens L, El-Khuffash A. Longitudinal Assessment of Left and Right Myocardial Function in Preterm Infants Using Strain and Strain Rate Imaging. Neonatology 2016;109:69-75. [Crossref] [PubMed]


