Radiation-induced gray matter atrophy in patients with nasopharyngeal carcinoma after intensity modulated radiotherapy: a MRI magnetic resonance imaging voxel-based morphometry study
Introduction
Nasopharyngeal carcinoma (NPC) ranks the third most common malignant tumor among men in certain regions of East Asia, and it has a high incidence rate of 15 to 50 per 100,000 (1). Radiotherapy (RT) has long been adopted as the standard radical treatment method. However, when the skull base was infiltrated by the tumor and the superior retropharyngeal lymph nodes were treated at the same time, bilateral temporal lobes (TLs) of NPC patients’ were routinely exposed to high doses of radiation. The long-term survival chance for these patients usually exceeds 70%, and the potential for intellectual impairment and cognitive deficits such as impaired learning and memory can be observed after a few months to few years post-irradiation (2-4).
For decades, 2D-radiotherapy (2D-RT) technique has been used for the treatment of NPC (5). Compared with 2D-RT, intensity modulated radiotherapy (IMRT) for NPC further improves the precision of RT delivery (6). IMRT differs completely from 2D-RT in terms of plan optimization, dose delivery and dose escalation, and enables optimal dose distribution in organs at risk. Being effective in reducing the higher doses of TLs, IMRT is expected to reduce the incidence of radiation induced brain injury. The radiological manifestations of IMRT-induced TL necrosis were less severe than those of 2D-RT (7). However, generation of highly conformal plans with steep fall-off gradients may come at the expense of significant doses to non-delineated extra-target organs (7). The incidence of cognitive impairment and memory impairment among patients is common (4). It was reported that the average doses in hippocampus were up to 30.27 Gy (range: 19.08–47.99 Gy) in NPCs with advanced (T3–4) primary tumor and a maximum dose exceeding 70 Gy was seen in 30% of cases (8). While another study of brain RT in glioma patients indicated that mean hippocampus volume was significantly reduced one year after exposure in the high-dose group (>40 Gy). Injury to the sub-granular zone of the hippocampus dentate gyrus is considered to be the main cause of memory impairment. Therefore, we hypothesized that there would be a decrease in volume of the hippocampus in NPC patients after delivery of IMRT, especially in the hippocampal head which is usually exposed to the higher dose. Voxel-based morphometry (VBM) is well known as a suitable method for assessing gray matter (GM) volume alterations (9). This method was used widely in brain studies related to cognitive changes and also psychiatric disorders (1-4). Recently, a study focused on the NPC patients also applied this method (10). With consideration of these previous studies, we made a further step and performed a research by using VBM to investigate RT induced GM volume differences between NPC patients who received RT (only IMRT) and those who did not. MRI is the most important for radiation therapy planning and monitoring for head and neck cancers (11).
Methods
The ethics institutional review board of Zhejiang Cancer Hospital approved the protocols for data collection and analyses. All the methods described here were performed in accordance with the relevant guidelines and regulations. Informed consent forms were signed by all patients.
Patients
Ninety-six NPC patients were included between November 2016 and July 2017 at the Zhejiang Cancer Hospital, Hangzhou, China. The after-RT group had 40 patients (32 males, mean age = 49.3±11.6 years, range: 23–72 years) who were diagnosed as NPC on the basis of histopathology, and had completed RT for more than 12months ago. T stage distribution for the after-RT group was T3 (n=21) and T4 (n=19). The T stage of NPC was defined according to American Joint Committee (AJCC) on cancer staging system (7th edition). The pre-RT group consisted of 56 patients (40 males, mean age = 47.5±11.6 years, range: 20–71 years) with newly diagnosed NPC and had not received RT.
Patients with an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate bone marrow, renal, and hepatic functions were eligible for this study. Patients with a prior (i.e., within 5 years) or synchronous malignancy were excluded. In addition, exclusion criteria for patients were as follows: medical history of central nervous system, cognitive or psychological diseases, intracranial invasion, brain tumors or metastases, severer systematic diseases, prior substantial head trauma, positive human immunodeficiency virus status, bad vision, other major medical illnesses, alcoholism, left-handedness or not suitable for MRI scans.
MRI protocol and data preprocessing
All the patients underwent the same protocol of T1-weighted 3D structural MRI scan using a 3.0 T scanner (Siemens, Verio, Germany). Images were acquired approximately parallel to the AC-PC line. Acquisition parameters were as follows: TR/TE =13/3.4 ms, flip angle =25°, field of view =240×240 mm2, slices numbers =144, matrix size =256×256, and the size for each voxel was 0.9375×0.9375×1 mm3. Head of the patients was stabilized with a foam cushion to minimize the motions during imaging.
T1-weighted images were preprocessed and analyzed using VBM8 toolbox in the SPM8 software (Welcome Department of Imaging Neuroscience Group, London, UK). Firstly, each T1-weighted structural scan was spatially normalized into the stereotactic space by co-registering with the standard MNI152 brain template. Then tissue maps corresponding to GM, white matter, and cerebrospinal fluid were extracted from the co-registered image. Finally, images were smoothed using a Gaussian kernel of 8 mm (FWHM).
Radiation therapy
All patients received continuous definitive RT by using IMRT with a prescription dose of 68.8 or 70.4 Gy in nasopharyngeal region and retropharyngeal region [for patients with positive retropharyngeal node(s)]. The design of the IMRT plan was based on previous studies (12-15).
The RT plan was designed using RayStation 4.0v (RaySearch Laboratories AB). The dosimetric parameters of the atrophic region on T1WI will be acquired using the mean dose (Dmean) of regions of interest (ROI) that was delineated by 1 radiologist and 1 radiation therapist on T1WI fused with CT. The equivalent uniform dose (EUD) was calculated by the following equation:
The equation meant to sum up the doses of dose-volume histogram (DVH) (vj, Dj), with αɛ (1, ∞). According to the references, α was assigned as 5 for brain tissue.
Chemotherapy
All patients in after-RT group received 2–3 cycles of TP or PF (docetaxel + cisplatin/nedaplatin, fluorouracil + cisplatin/nedaplatin) induction chemotherapy, followed by IMRT and cisplatin/nedaplatin concurrent chemotherapy.
Statistical analysis
After preprocessing, we analyzed the correlation between age and GM, voxel-wise comparisons of GM volume were performed between the pre-RT group and after-RT group using two-sample t-test based on the general linear model to determine the regions where the GM thickness changed. The hippocampal head was specially defined as the anterior hippocampus constituting of 35% of slices (13). Both age and gender have been included as covariants. Statistical significance was determined by a voxel-level statistical threshold (P<0.001) with AlphaSim correction for multiple comparisons (minimum cluster size of 157 voxels). AlphaSim correction was performed using DPABI software (DPABI_V1.3_150710, http://rfmri.org/dpabi).
Mean doses (Dmean) from each atrophic GM are presented as mean ± standard deviation and all statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA). One way ANOVA was used to evaluate the Dmean among the groups. Pearson’s correlation was used to evaluate the correlations between the GM volume in any brain regions that showed the difference and the mean dose of the corresponding position. P value <0.05 was considered as statistical significance.
Results
Correlation analysis in pre-RT group showed negative correlation between the whole brain GM with age (r=−0.463, P<0.001), and also negative correlation with age in after-RT group (r=−0.376, P=0.017). No significant differences were detected between the age and gender of the two groups (P>0.05).
GM volume between pre- and after- RT NPC patients
Voxel-wise comparisons of GM volume were performed between the pre-RT group and after-RT group using two-sample t-tests based on the general linear model. Results revealed that the GM volume decreased in the after-RT group in these brain regions: the right pulvinar (x=20, y=−30, z=6), the left hippocampus (x=−26, y=−16, z=−14) and the right middle temporal gyrus (MTG; x=53, y=−28, z=−2), compared to pre-RT group (P<0.001, AlphaSim correction, cluster size ≥157). The results are presented in Table 1 and Figures 1,2. In the corrected results no region was detected showing GM volume increased.

Full table

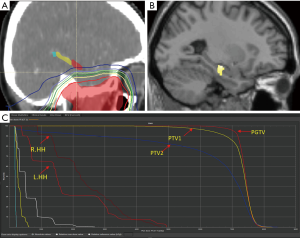
Dosimetry analysis of atrophic GM
Because image fuse between CT and T1WI was not successful in 4 cases, dosimetry analysis of 36 patients was performed. IMRT of 7–8 fields was performed under actual situation. The diagram of the exposed dose and the dose-volume histogram (DVH) of lateral hippocampal head is demonstrated in Figure 2. Dmean of different regions are: 1,829.3±905.3 cGy for left hippocampal head (L.HH), 2,129.2±1198 cGy for right hippocampal head (R.HH), 314.8±200.2 cGy for right pulvinar (R.PV), 79.6±30.5 cGy for right MTG (Figure 3). The Dmean for L.HH and R.HH are significantly higher than other parts of the brain (P<0.001). There were no significant differences between the Dmean for L.HH and R.HH (P=0.99). No significant correlations between the GM volume in any brain regions and the mean dose of corresponding position were observed (P>0.05).

Discussion
Most NPC patients after IMRT showed little change in their brain tissue according to the conventional MRI, but they may still suffer the radiation-induced cognitive impairment (16). It is necessary to evaluate radiation-induced injury in patients with MRI normal-appearing brain tissue and provide neuroimaging biomarkers to facilitate clinical management. This study shows VBM could demonstrate regional difference of GM volume in MRI normal-appearing GM between post-radiated NPC patients and newly diagnosed counterparts awaiting RT. This study shows that compared to pre-RT group, cortical volume was significantly decreased in specific regions of after-RT group including left hippocampus, right pulvinar and right MTG (P<0.001, AlphaSim correction, cluster size ≥157); and there was no linear correlation between regional GM volume loss and Dmean of corresponding position (P>0.05).
Lv et al. (10) reported that NPCs had GM volume significantly decreased in the bilateral superior temporal gyrus, left MTG, right fusiform gyrus, right precentral gyrus, and right inferior parietal lobule 1–20 months after RT (P<0.001, uncorrected, cluster size >100 voxels). The result of GM volume decrease in our study is in line with that study. However, as Lv et al.’s study included a certain number of T1–2 NPC patients thus leading to high dose disparities in hippocampus, no change was found in the hippocampus in their study. To our knowledge, ours is the first study that reveals the findings of GM volume deficit in hippocampus of NPC patients who received IMRT and the imaging evidence confirming that the RT damage to hippocampus. As is known, left hippocampus plays a particular role in verbal memory (17). The RTOG 0933 study (18), a single arm phase II multi-institutional trial, was conducted to evaluate the efficiency of the hippocampus sparing in brain metastasis patients who underwent whole-brain radiotherapy (WBRT). Their results revealed significantly higher Hopkins Verbal Learning Test (HVLT) in patients receiving hippocampal-avoidance WBRT (HA-WBRT) compared to the historical control. Numerous studies have indicated that neurogenesis constantly takes place in the human system, while neural precursor cells and new neurons are essential in maintaining learning and memory. New neurons are generated in a few regions of the adult brain, including subventricular zone (SVZ), and the granular cell subregion of the striatum and dentate gyrus (19). The hippocampus, especially CA1 subfields and gyrus dentate, is the area of the brain that is sensitive to radiation (20). Even lower dose radiation can cause significant changes in the spinal volume and morphology of the hippocampus formation (21,22), leading to reduced dendritic branching of granule cells located in gyrus dentatus (2,22). The pathological changes, which present as loss of neural progenitor cells by immunohistochemical assessment, showed widespread GM volume deficits in the developing animals. A variety of studies indicate that changes in oligodendrocytes may result in radiation-induced neural deficits and cognitive dysfunction, suggesting that radiation can trigger the proliferation of microglial and oligodendrocyte precursor cells in the hippocampus. It remains unclear whether radiation-induced death of oligodendrocytes is another reason for hippocampal reduction (23). In addition, the influence of chemotherapy cannot be excluded in extensive GM deficits. According to one previous study, platinum-based chemotherapy exposure reduced dendritic branching and spine volume, and induced apoptosis of neurons and NSC within the hippocampal formation (24). The alteration of GM volume was observed in breast cancer because some cytotoxic agents, mainly including 5-FU, thiotepa and cyclophosphamide, can cross over the blood brain barrier, and disrupt cell division in the brain regions that are critical for memory and learning (25). Docetaxel might cause cognitive impairment (26), but the neurotoxic effects of docetaxel tend to be a short-term impact.
In this study, the dose constraint for partial brain was 72 Gy (max), avoiding >2 Gy/fraction or hyperfractionation. The dose constraint for the whole brain volume was 12 Gy <5–10 cm3; however, there was no dose constraint for hippocampus and other specific region. The different dosages delivered to different regions were influenced by the RT dose to the tumor and the distance between tumor and specific region under the constraint mentioned above.
Dmean for L.HH and R.HH was significantly higher compared to other parts of the brain (P<0.001). It’s also interesting that only left hippocampus showed notable atrophy. We speculate that hippocampus on two sides show different sensitivity to radiation and chemotherapy toxicity due to subtle lateralized differences in cytoarchitecture. The hippocampal formation is more complex in the left side than the right, as it has been reported the total number of granule cells of dendritic segments is larger in the left than that in the right, suggesting that they have a larger receptor surface (17). Radiation or chemotherapy induces toxicity shows a decrease of dendritic branching and spine volume, which are more obvious in the left hippocampus than in its counterpart. However, although the right hippocampus showed no obvious atrophy in our study, it doesn’t mean that the right hippocampus was undamaged. The injury of right hippocampus probably exists but not demonstrable with our methodology.
Our results also demonstrated other GM volume deficits in brain regions such as right MTG and right pulvinar. As these regions are not exposed to the high dose of RT, the GM volume deficits in these areas could not be interpreted as radiation induced injury. There are a few possible reasons. It is likely that the GM volume deficits in these cortices may be secondary changes. The thalamus is an integrative hub for functional brain networks, which can be divided into first-order and higher-order thalamic nuclei. Higher-order thalamic nuclei, such as pulvinar nuclei, has both reciprocal and nonreciprocal connections with multiple cortical regions, including TL. Significant correlations between ipsilateral hippocampal volume and volumes of both ipsilateral and contralateral thalamotemporal segments have been observed (27). It is possible that the GM deficits of MTG could be caused by these connections. The supramarginal gyrus also has functional connectivity with thalamus, which has been considered as a gateway of sensory input by transferring information from the sensory periphery to MTG (28). In addition, GM volume deficits of MTG and pulvinar may be affected by chemotherapy which requires further studies.
The main limitation of this study is that the effect of RT dosage and timing of GM volume change could not be studied. Neuropsychological test data were not available.
In conclusion, our results demonstrate imaging evidence of atrophy of the hippocampus and other multiple GM deficits in NPC patients treated with IMRT.
Acknowledgements
Funding: This study was supported by grant from Medical and Health Science and Technology Program of Zhejiang Province (NO. 2013RCA007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics institutional review board of Zhejiang Cancer Hospital approved the protocols for data collection and analyses. All the methods described here were performed in accordance with the relevant guidelines and regulations. Informed consent forms were signed by all patients.
References
- Li Y, Shi X, Rong X, Peng Y, Tang Y. Neurosurgery and prognosis in patients with radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy: a follow-up study. Radiat Oncol 2013;8:88. [Crossref] [PubMed]
- Hellström NAK, Björk-Eriksson T, Blomgren K, Kuhn HG. Differential Recovery of Neural Stem Cells in the Subventricular Zone and Dentate Gyrus After Ionizing Radiation. Stem Cells 2009;27:634-41. [Crossref] [PubMed]
- Li H, Li JP, Lin CG, Liu XW, Geng ZJ, Mo YX, Zhang R, Xie CM. An experimental study on acute brain radiation injury: dynamic changes in proton magnetic resonance spectroscopy and the correlation with histopathology. Eur J Radiol 2012;81:3496-503. [Crossref] [PubMed]
- Mao YP, Zhou GQ, Liu LZ, Guo R, Sun Y, Li L, Lin AH, Zeng MS, Kang TB, Jia WH, Shao JY, Mai HQ, Ma J. Comparison of radiological and clinical features of temporal lobe necrosis in nasopharyngeal carcinoma patients treated with 2D radiotherapy or intensity-modulated radiotherapy. Br J Cancer 2014;110:2633-9. [Crossref] [PubMed]
- Su SF, Huang Y, Xiao WW, Huang SM, Han F, Xie CM, Lu TX. Clinical and dosimetric characteristics of temporal lobe injury following intensity modulated radiotherapy of nasopharyngeal carcinoma. Radiother Oncol 2012;104:312-6. [Crossref] [PubMed]
- Tan W, Ye J, Xu R, Li X, He W, Wang X, Li Y, Hu D. The tumor shape changes of nasopharyngeal cancer during chemoradiotherapy: the estimated margin to cover the geometrical variation. Quant Imaging Med Surg 2016;6:115-24. [Crossref] [PubMed]
- Rosenthal DI, Chambers MS, Fuller CD, Rebueno NCS, Garcia J, Kies MS, Morrison WH, Ang KK, Garden AS. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2008;72:747-55. [Crossref] [PubMed]
- Khodayari B, Michaud AL, Stanic S, Wooten OH, Dublin A, Purdy JA, Chen AM. Evaluation of hippocampus dose for patients undergoing intensity-modulated radiotherapy for nasopharyngeal carcinoma. Br J Radiol 2014;87. [Crossref] [PubMed]
- Martin P, Bender B, Focke NK. Post-processing of structural MRI for individualized diagnostics. Quant Imaging Med Surg 2015;5:188-203. [PubMed]
- Lv XF, Zheng XL, Zhang WD, Liu LZ, Zhang YM, Chen MY, Li L. Radiation-induced changes in normal-appearing gray matter in patients with nasopharyngeal carcinoma: A magnetic resonance imaging voxel-based morphometry study. Neuroradiology 2014;56:423-30. [Crossref] [PubMed]
- Yuan J, Lo G, King AD. Functional magnetic resonance imaging techniques and their development for radiation therapy planning and monitoring in the head and neck cancers. Quant Imaging Med Surg 2016;6:430-448. [Crossref] [PubMed]
- Yu Z, Luo W, Zhou QC, Zhang QH, Kang DH, Liu MZ. Impact of changing gross tumor volume delineation of intensity-modulated radiotherapy on the dose distribution and clinical treatment outcome after induction chemotherapy for the primary locoregionally advanced nasopharyngeal carcinoma. Ai Zheng 2009;28:1132-7. [Crossref] [PubMed]
- Lee AWM, Lau KY, Hung WM, Ng WT, Lee MCH, Choi CW, Chan CC, Tung R, Cheng PT, Yau TK. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol 2008;87:204-10. [Crossref] [PubMed]
- Li K, Yang L, Xin P, Chen Y, Hu QY, Chen XZ, Chen M. Impact of dose volume parameters and clinical factors on acute radiation oral mucositis for locally advanced nasopharyngeal carcinoma patients treated with concurrent intensity-modulated radiation therapy and chemoradiotherapy. Oral Oncol 2017;72:32-7. [Crossref] [PubMed]
- Chaikh A, Balosso J. Agreement between gamma passing rates using computed tomography in radiotherapy and secondary cancer risk prediction from more advanced dose calculated models. Quant Imaging Med Surg. 2017;7:292-298. [Crossref] [PubMed]
- Lin J, Lv X, Niu M, Liu L, Chen J, Xie F, Zhong M, Qiu S, Li L, Huang R. Radiation-induced abnormal cortical thickness in patients with nasopharyngeal carcinoma after radiotherapy. NeuroImage Clin 2017;14:610-21. [Crossref] [PubMed]
- Sá MJ, Ruela C, Madeira MD. Dendritic right/left asymmetries in the neurons of the human hippocampal formation: A quantitative Golgi study. Arq Neuropsiquiatr 2007;65:1105-13. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Lazarov O, Hollands C. Hippocampal neurogenesis: learning to remember. Prog Neurobiol 2016;138-140:1-18. [Crossref] [PubMed]
- Hua C, Merchant TE, Gajjar A, Broniscer A, Zhang Y, Li Y, Glenn GR, Kun LE, Ogg RJ. Brain tumor therapy-induced changes in normal-appearing brainstem measured with longitudinal diffusion tensor imaging. Int J Radiat Oncol Biol Phys 2012;82:2047-54. [Crossref] [PubMed]
- Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One 2012;7. [Crossref] [PubMed]
- Park MK, Kim S, Jung U, Kim I, Kim JK, Roh C. Effect of acute and fractionated irradiation on hippocampal neurogenesis. Molecules 2012;17:9462-8. [Crossref] [PubMed]
- Hua K, Schindler MK, McQuail JA, Forbes ME, Riddle DR. Regionally Distinct Responses of Microglia and Glial Progenitor Cells to Whole Brain Irradiation in Adult and Aging Rats. PLoS One 2012;7. [Crossref] [PubMed]
- John T, Lomeli N, Bota DA. Systemic cisplatin exposure during infancy and adolescence causes impaired cognitive function in adulthood. Behav Brain Res 2017;319:200-6. [Crossref] [PubMed]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp 2012;33:2971-83. [Crossref] [PubMed]
- Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB. Cognitive impact of cytotoxic agents in mice. Psychopharmacology (Berl) 2015;232:17-37. [Crossref] [PubMed]
- Keller SS, O’Muircheartaigh J, Traynor C, Towgood K, Barker GJ, Richardson MP. Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia 2014;55:306-15. [Crossref] [PubMed]
- Jung J, Kim S, Cho H, Nam K. Structural and functional correlates for language efficiency in auditory word processing. PLoS One 2017;12. [Crossref] [PubMed]


