Type II endoleak model creation and intraoperative aneurysmal sac embolization with n-butyl cyanoacrylate-lipiodol-ethanol mixture (NLE) in swine
Introduction
Endovascular abdominal aortic repair (EVAR) is widely performed as a less-invasive alternative method for patients with abdominal aortic aneurysm (AAA) who are not candidates for conventional surgical open repair. EVAR has an early survival benefit compared to open repair, including shorter hospital stay, less blood loss, shorter surgical duration, and lower morbidity and mortality rates (1-3). However, it has been recently reported that EVAR has no long-term survival benefit compared to open repair, and that there are also significantly higher risks of reintervention and aneurysm rupture after EVAR than open repair (4-6). Patients who had EVAR need lifelong surveillance after EVAR. Endoleak and aneurysm sac expansion after EVAR are significant problems that need to be overcome. Type II endoleak often spontaneously disappear, but 10–25% remain (7-10). Persistent type II endoleak is strongly associated with aneurysm sac enlargement (11-13). Re-intervention is mandatory for endoleak with sac expansion.
Intraoperative aneurysmal sac embolization during EVAR can be one of the option to prevent type II endoleak (14-16). N-butyl cyanoacrylate (NBCA, Histoacryl, B. Braun, Melsungen, Germany) is one of liquid embolic materials. A mixture of NBCA with Lipiodol (Guerbet, Villepinte, France) and ethanol (Fuso Pharmaceutical Industries Ltd, Osaka, Japan) (NBCA-lipiodol-ethanol; NLE) is less adhesive embolic material than NBCA (17). The aim of this study was to evaluate the feasibility of type II endoleak model creation in swine and efficacy of intraoperative AAA sac embolization during EVAR using NLE for type II endoleak.
Methods
The approval of the Institutional Committee on Research-Animal Care was obtained before initiation of the study.
Six healthy female swine weighing 48–55 kg (mean body weight 53.5 kg) were prepared. Two swine were used for a control group, and 4 swine for NLE embolization group. Pre-anesthesia comprised 5 mg/kg ketamine and 80 µg/kg medetomidine, and general anesthesia was maintained with isoflurane gas via tracheal intubation. Cardiac and pulmonary parameters were monitored throughout the procedures. The technique of type II endoleak model creation consisted of three steps. That is, the first step is creation of an abdominal aneurysm using inferior vena cava (IVC). The second step is anastomosis between renal artery and aneurysm, and the final step is stent-graft placement in the abdominal aorta. First, laparotomy was performed and abdominal aorta was surgically exposed. Abdominal aneurysm was created by using IVC. A length of IVC of approximately 3 cm was removed and then sutured to an incision in the infrarenal abdominal aorta, under interruption of abdominal aortic blood flow. We created a total of 6 AAAs in 6 swine. The long × short diameters ranged from 42 mm × 22 mm to 56 mm × 26 mm (mean, 47.5 mm × 26.2 mm). Next, the left renal artery was selected as an artery causing type II endoleak. Left nephrectomy was performed and then end-to-side anastomosis between the left renal artery and AAA was created (Figure 1A).
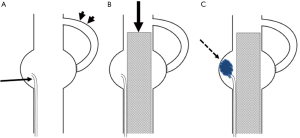
A 6-Fr sheath (Long sheath, Terumo, Gifu, Japan) was placed in both surgically exposed femoral arteries after 0.035-inch guidewire (Terumo) insertion. A 4-Fr pig tail catheter (Medikit, Tokyo, Japan) was advanced into abdominal aorta via right femoral artery and aortography was performed. After that, a 2.7-Fr microcatheter (Carnelian; Tokai Medical, Aichi, Japan) was inserted into the AAA using a 0.014-inch guidewire (GT wire; Terumo). Finally, after 0.035-inch stiff guidewire (Cook Inc., Bloomington, IN, USA) was inserted via the left femoral artery, a 12-Fr sheath with the stent-graft (Zenith® iliac leg extension 8 mm diameter, 37 mm long) (Cook, USA) was inserted and deployed into the abdominal aorta, covering the AAA (Figure 1B). Aortography was performed to confirm the type II endoleak from the anastomosed left renal artery.
NLE was prepared by mixing NBCA, Lipiodol, and ethanol at a ratio of 1:5:1 by use of a three-way stopcock. The components were mixed thoroughly before injection.
As a control group, stent-graft deployment was performed without AAA sac embolization in two swine. For NLE embolization group, after stent-graft deployment, AAA sac was embolized with NLE in four swine. Before injection of NLE, we estimated the volume of the AAA sac by test injection of contrast medium. NLE was slowly injected via the microcatheter inserted into AAA sac under fluoroscopic control (Figure 1C). NLE was injected until the AAA sac was completely filled in with NLE. After the AAA sac was filled in with NLE, the microcatheter was removed. And then aortography was performed to confirm the presence or absence of flow into the aneurysm and the type II endoleak after embolization. Three days later, aortography was performed again to depict type II endoleak.
After the final aortography, the swine are euthanized to extract the aneurysms. After the formalin-fixation, Hematoxylin-Eosin (HE) staining was performed to confirm the thrombosis of the aneurysm, and observe the extent of the histopathological inflammatory changes of vessel wall.
Results
The AAA sac and type II endoleak model were successfully created in all cases.
In the NLE embolization group, AAA sac was completely filled in with NLE (Figure 2). Endoleak disappeared immediately after the procedure and no recurrence was observed three days after the procedure (Table 1). AAA sac was occupied with organized thrombus, degenerate blood cells, and embolic material (Figure 3). Inflammatory changes were recognized in aneurysmal sac wall (Figure 4A).
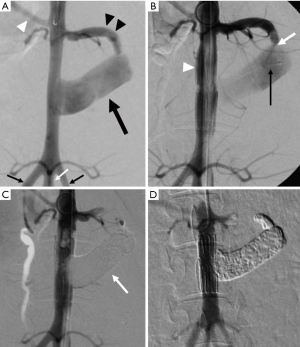

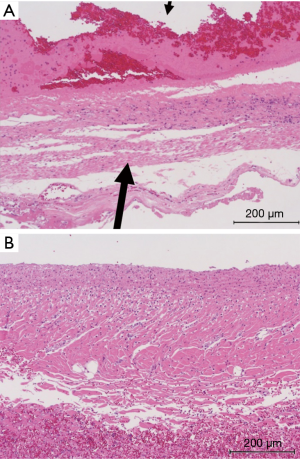
In the control group, type II endoleak persisted three days after the procedure (Table 1). Inflammatory changes were almost not recognized in aneurysmal sac wall (Figure 4B). There was much more infiltration of inflammatory leukocytes into the vessel wall in the NLE embolization group than in the control group as shown with HE stain specimens.

Full table
Discussion
Endoleak after EVAR are classified into four types (18). Type I endoleak is characterized by arterial blood flow into the aneurysmal sac from the proximal or distal attachment site of the stent-graft as a result of inadequate fixation. Type II endoleak, the most common type, is characterized by blood flow in a retrograde manner into the aneurysmal sac through patent branch vessels of the abdominal aorta, such as lumbar artery (LA) or inferior mesenteric artery (IMA). Type III endoleak is characterized by blood leak from a modular disconnection or graft fabric tear. Type IV endoleak is characterized by sac perfusion from blood permeation through the graft material, which results from excessive graft porosity. The mechanism of type II endoleak is thought to be that backflow from the aortic branch vessel occurs by the sac pressure reduction by covering the aneurysm with the stent-graft. We succeeded in creating a type II endoleak model by anastomosing the renal artery with the aneurysm. After stent-graft placement into the abdominal aorta covering the AAA sac, a type II endoleak model was achieved.
NBCA is a liquid adhesive composed of monomers, and polymerizes into a solid material following contact with anions within blood. To achieve radio-opacity, NBCA is usually mixed with iodized oil (Lipiodol). Polymerization time depends on the dilution ratio of these two components (19-22). NBCA is increasingly being used to treat acute arterial hemorrhage and pseudoaneurysms because its rapid polymerization in blood results in complete and instantaneous occlusion of the bleeding vessel (23-25). However, because of the strong adhesive properties of NBCA, NBCA has some concerns such as adhesion to the catheter and vessel wall, and early polymerization of NBCA within the catheter and proximal portion of the target vessel, leading to incomplete embolization.
The characteristics of NLE have been shown to be different from those of the NBCA-lipiodol mixture (NL) (17,26-28). NLE may have multiple advantages over NL for embolization, such as less adhesive properties to the catheter and less damage features to the vascular wall (26,27). NLE doesn’t adhere to the balloon catheter and showed the feasibility of packing a wide-neck aneurysm (26,27). Ishikawa et al. reported that NLE showed consistent and reproducible complete embolization with flow control compared to NL and was stable after balloon deflation (28).
In this study, NLE with a ratio of 1:5:1 was used as the embolic material. We determined the ratio of the components of the embolic material based on our clinical experiences (29). Our mixing ratio enabled us to achieve complete packing of the AAA sac. We believe that this mixing ratio was appropriate for AAA sac embolization, considering adhesion of NLE glue and safety, although the optimum ratio of NLE may still be controversial. The optimum ratio of NLE might need to be adjusted depending on the size of the AAA sac and its flow. The behavior of NLE was safely monitored under fluoroscopic guidance. NLE can be slowly injected in a controlled manner, and the procedure does not require rapid removal of the catheter, unlike with standard NL mixture. NLE induced thrombosis of the aneurysmal sac, and this procedure eliminated the type II endoleak.
There are some reports on embolization for the sac and endoleak using other liquid embolic materials such as thrombin or Onyx (LES, Covidien, Plymouth, MN, USA) (14,30). However, the difficulty of monitoring thrombin progression may be at risk of non-target vessel embolization. Furthermore, the injected thrombin is usually completely reabsorbed within several days as a result of fibrinolysis and tissue plasminogen reactions (31), possibly leading to less good occlusive properties. Onyx needs more time for preparation and is more painful at the time of injection. Additionally, it has more cytotoxic effect than NBCA due to dimethyl sulfoxide (DMSO) (32). Intraoperative aneurysmal sac embolization needs too much liquid embolic material, so a large amount of onyx has concern about toxicity. Therefore, we think the use of NLE is quite easier and better.
The limitation of our study is to have used the left renal artery to create a type II endoleak model. The renal artery provides more blood flow into the sac than thin aortic branches such as LA and IMA which commonly cause type II endoleak. That is, the hemodynamic of this endoleak model differs slightly from that of the naturally occurring endoleak. However, this study showed that even endoleak caused by such thick artery could be embolized by NLE. Another limitation is the relatively short follow-up period after endovascular procedure. We could not evaluate about the recurrence of endoleak after long term follow-up from the viewpoint of animal welfare.
In conclusion, this experimental study suggests that creation of a type II endoleak model in swine is feasible and that intraoperative AAA sac embolization with NLE during EVAR might reduce the occurrence of type II endoleak.
Acknowledgements
Funding: This work was supported by JSPS KAKENHI Grant Number JP15K09969.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This experimental study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee on research animal care.
References
- Matsumura JS, Brewster DC, Makaroun MS, Naftel DC. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg 2003;37:262-71. [Crossref] [PubMed]
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8. [Crossref] [PubMed]
- Brewster DC, Jones JE, Chung TK, Lamuraglia GM, Kwolek CJ, Watkins MT, Hodgman TM, Cambria RP. Long-term outcomes after endovascular abdominal aortic aneurysm repair: the first decade. Ann Surg 2006;244:426-38. [PubMed]
- Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 2013;100:863-72. [Crossref] [PubMed]
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. [Crossref] [PubMed]
- Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin JP, Greenhalgh RM. EVAR-1, DREAM, OVER and ACE Trialists. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br J Surg 2017;104:166-78. [Crossref] [PubMed]
- Makaroun M, Zajko A, Sugimoto H, Eskandari M, Webster M. Fate of endoleaks after endoluminal repair of abdominal aortic aneurysms with the EVT device. Eur J Vasc Endovasc Surg 1999;18:185-90. [Crossref] [PubMed]
- Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, Buth J, Chuter TA, Fairman RM, Gilling-Smith G, Harris PL, Hodgson KJ, Hopkinson BR, Ivancev K, Katzen BT, Lawrence-Brown M, Meier GH, Malina M, Makaroun MS, Parodi JC, Richter GM, Rubin GD, Stelter WJ, White GH, White RA, Wisselink W, Zarins CK. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002;35:1029-35. [Crossref] [PubMed]
- Greenberg RK, Chuter TA, Sternbergh WC 3rd, Fearnot NE. Zenith Investigators. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg 2004;39:1209-18. [Crossref] [PubMed]
- Nakai M, Sato M, Sato H, Sakaguchi H, Tanaka F, Ikoma A, Sanda H, Nakata K, Minamiguchi H, Kawai N, Sonomura T, Nishimura Y, Okamura Y. Midterm results of endovascular abdominal aortic aneurysm repair: comparison of instruction-for-use (IFU) cases and non-IFU cases. Jpn J Radiol 2013;31:585-92. [Crossref] [PubMed]
- Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, Hodgman TM, Cambria RP. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 2007;46:1-8. [Crossref] [PubMed]
- Higashiura W, Greenberg RK, Katz E, Geiger L, Bathurst S. Predictive factors, morphologic effects, and proposed treatment paradigm for type II endoleaks after repair of infrarenal abdominal aortic aneurysms. J Vasc Interv Radiol 2007;18:975-81. [Crossref] [PubMed]
- van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J, Collaborators EUROSTAR. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg 2004;27:128-37. [Crossref] [PubMed]
- Zanchetta M, Faresin F, Pedon L, Ronsivalle S. Intraoperative intrasac thrombin injection to prevent type II endoleak after endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2007;14:176-83. [Crossref] [PubMed]
- Ward TJ, Cohen S, Fischman AM, Kim E, Nowakowski FS, Ellozy SH, Faries PL, Marin ML, Lookstein RA. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol 2013;24:49-55. [Crossref] [PubMed]
- Nakai M, Ikoma A, Sato M, Sato H, Nishimura Y, Okamura Y. Prophylactic intraoperative embolization of abdominal aortic aneurysm sacs using n-butyl cyanoacrylate/lipiodol/ethanol mixture with proximal neck aortic balloon occlusion during endovascular abdominal aortic repair. J Vasc Interv Radiol 2016;27:954-60. [Crossref] [PubMed]
- Kawai N, Sato M, Minamiguchi H, Ikoma A, Sanda H, Nakata K, Tanaka F, Nakai M, Sonomura T. Basic study of a mixture of N-butyl cyanoacrylate, ethanol, and lipiodol as a new embolic material. J Vasc Interv Radiol 2012;23:1516-21. [Crossref] [PubMed]
- Bashir MR, Ferral H, Jacobs C, McCarthy W, Goldin M. Endoleaks after endovascular abdominal aortic aneurysm repair: management strategies according to CT findings. AJR Am J Roentgenol 2009;192. [Crossref] [PubMed]
- American Society for Gastrointestinal Endoscopy Assessment Committee Technology. Status evaluation report: tissue adhesives and fibrin glues. Gastrointest Endosc 2004;60:327-33. [Crossref]
- Stoesslein F, Ditscherlein G, Romaniuk PA. Experimental studies on new liquid embolization mixtures (histoacryl-Lipiodol, histoacryl-panthopaque). Cardiovasc Intervent Radiol 1982;5:264-7. [Crossref] [PubMed]
- Spiegel SM, Vinuela F, Goldwasser JM, Fox AJ, Pelz DM. Adjusting the polymerization time of isobutyl-2 cyanoacrylate. Am J Neuroradiol 1986;7:109-12. [PubMed]
- Gounis MJ, Lieber BB, Wakhloo AK, Siekmann R, Hopkins LN. Effect of glacial acetic acid and ethiodized oil concentration on embolization with n-butyl 2-cyanoacrylate: an in vivo investigation. AJNR Am J Neuroradiol 2002;23:938-44. [PubMed]
- Kish JW, Katz MD, Marx MV, Harrell DS, Hanks SE. N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. J Vasc Interv Radiol 2004;15:689-95. [Crossref] [PubMed]
- Yonemitsu T, Kawai N, Sato M, Tanihata H, Takasaka I, Nakai M, Minamiguchi H, Sahara S, Iwasaki Y, Shima Y, Shinozaki M, Naka T, Shinozaki M. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J Vasc Interv Radiol 2009;20:1176-87. [Crossref] [PubMed]
- Nakai M, Sato M, Sanda H, Ikoma A, Kawai N, Minamiguchi H, Nakata K, Tanaka T, Sonomura T. Percutaneous fluoroscopically guided n-butyl cyanoacrylate (NBCA) injection for iatrogenic femoral arterial pseudoaneurysm under temporary balloon occlusion of arterial blood flow. Jpn J Radiol 2012;30:365-9. [Crossref] [PubMed]
- Tanaka F, Kawai N, Sato M, Minamiguchi H, Nakai M, Nakata K, Sanda H, Sonomura T. Balloon-assisted packing of wide-neck aneurysms with a mixture of n-butyl cyanoacrylate, Lipiodol, and ethanol: an experimental study. Jpn J Radiol 2015;33:517-22. [Crossref] [PubMed]
- Tanaka F, Kawai N, Sato M, Minamiguchi H, Nakai M, Nakata K, Sanda H, Sonomura T, Matuzaki I, Murata S. Effect of transcatheter arterial embolization with a mixture of n-butyl cyanoacrylate, lipiodol, and ethanol on the vascular wall: macroscopic and microscopic studies. Jpn J Radiol 2015;33:404-9. [Crossref] [PubMed]
- Ishikawa M, Horikawa M, Yamagami T, Uchida BT, Awai K, Kaufman JA. Embolization of Arteriovenous Malformations: Effect of Flow Control and Composition of n-Butyl-2 Cyanoacrylate and Iodized Oil Mixtures with and without Ethanol in an in Vitro Model. Radiology 2016;279:910-6. [Crossref] [PubMed]
- Nakai M, Ikoma A, Higashino N, Inagaki T, Sonomura T. Balloon-occluded retrograde transvenous obliteration of a gastric varix with the use of an n-butyl cyanoacrylate-lipiodol-ethanol mixture. J Vasc Interv Radiol. 2018;29:1325-7. [Crossref] [PubMed]
- Marcelin C, Le Bras Y, Petitpierre F, Midy D, Ducasse E, Grenier N, Cornelis F. Safety and efficacy of embolization using Onyx® of persistent type II endoleaks after abdominal endovascular aneurysm repair. Diagn Interv Imaging 2017;98:491-7. [Crossref] [PubMed]
- Muthu C, Maani J, Plank LD, Holden A, Hill A. Strategies to reduce the rate of type II endoleaks: routine intraoperative embolization of the inferior mesenteric artery and thrombin injection into the aneurysm sac. J Endovasc Ther 2007;14:661-8. [Crossref] [PubMed]
- Ozdol C, Turk CC, Hazer DB, Yildirim AE, Arat A, Atilla P, Muftuoglu S, Oruckaptan H. Comparison of the Toxicities of Ethylene Vinyl Alcohol Copolymer (EVOH) Preparations, Dimethyl Sulphoxide and N-Butyl 2-Cyanoacrylate on Cerebral Parenchyma in an Experimental Rabbit Model. Turk Neurosurg 2015;25:446-52. [PubMed]


