
Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism
Introduction
Computed tomography pulmonary angiography (CTPA) is currently the preferred imaging modality for diagnosis of suspected pulmonary embolism (PE). With improved spatial and temporal resolution available with modern CT scanners, CTPA has high diagnostic accuracy in the detection of segmental and subsegmental PE (1-3). However, the high radiation dose associated with CTPA is still a concern, given the high prevalence of PE and widespread use of less-invasive imaging for clinical diagnosis (1-5). Therefore, improvements in CT technique to minimize radiation dose are necessary.
A number of strategies have already been developed which include low tube voltage (kVp), use of iterative reconstruction (IR) for reducing image noise, and use of high-pitch protocols with fast speed CT scanners (6-13). Significant progress has been achieved with use of these dose-reduction strategies with radiation dose lowered to less than 2 mSv, according to some recent studies (14-16). Despite these promising results, further dose reduction by combining different parameters remains to be determined. Thus, the purpose of this study was to investigate the optimal CTPA protocols with use of different kVp and pitch values. Since it is unethical to scan patients with different CT protocols, we decided to use a patient-specific three-dimensional (3D) printed pulmonary artery model with simulation of PE in the pulmonary arteries. In our previous paper, we described how we developed a 3D printed pulmonary artery model and confirmed its accuracy and validity in replicating normal pulmonary arteries by testing different CT scanning parameters on it (17).
In this publication we describe how we extended our previous research by inserting thrombus in the pulmonary arteries to simulate PE, and scanning the model with different CTPA protocols. Although patient-specific 3D printed models have been reported in the literature with regard to their accuracy and usefulness in preoperative planning and simulation (18-23), to the best of our knowledge, this is the first study using a 3D printed pulmonary artery model with thrombus inside the arteries for determining optimal CTPA protocols.
Methods
Selection of sample case and image post-processing and segmentation
CTPA images of patients with suspected PE were retrospectively reviewed and one sample case with normal CTPA findings without any sign of PE was selected for generation of the pulmonary artery model with details provided in our previous study (17).
The same approach was used to perform image post-processing and segmentation of CTPA images as described previously (17). Figure 1 shows the steps that were undertaken to generate a 3D segmented volume file for 3D printing of the pulmonary artery lumen. The 3D model was printed using an online printing service, Shapeways (24). An elastoplastic material was used to print the model since it has similar properties to that of arterial wall (25).

3D printing of pulmonary artery model with simulation of thrombus
To simulate PE in the pulmonary arteries, animal blood clots which were obtained from a local butcher were inserted into the left and right main pulmonary arteries of the 3D printed model mimicking thrombus. To prevent the “thrombus” from moving during CT scans, the blood clots were large enough to be deployed in the main pulmonary arteries, thus remaining stable during the scans.
CTPA scanning protocols
The 3D printed pulmonary artery model with thrombi inside was placed in a plastic container which was filled with contrast medium to simulate contrast-enhanced CT examinations. The contrast medium OptirayTM 350 (Mallinckrodt Pty Ltd, NSW, Australia) was diluted to 7% with resulting CT attenuation of 200 HU similar to that of routine CTPA. CTPA scans were performed on a dual-source 128-slice CT scanner (Siemens Definition Flash, Siemens Healthcare, Forchheim, Germany) with beam collimation of 2×64×0.6 mm and gantry rotation of 330 ms. Tube current modulation was used for all scans while different kVp and pitch values were chosen (70, 80, 100 and 120 kVp and pitch of 0.9, 2.2 and 3.2), resulting in a total of 12 datasets. A slice thickness of 1.0 mm with a 0.5 mm reconstruction interval was applied to all images, resulting in the voxel size of 0.29×0.29×0.29 mm3 for volumetric data. All images were reconstructed with sinogram affirmed iterative reconstruction (SAFIRE, Siemens Healthcare) at a strength level of 3, and a tissue convolution kernel of I30f.
Image post-processing and visualization of PE
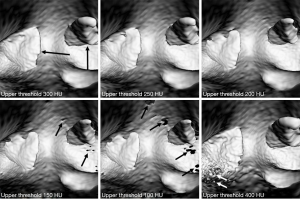
Two-dimensional (2D) images in digital imaging and communications in medicine (DICOM) format were transferred to a workstation with Analyze V 12.0 (AnalyzeDirect, Inc., Lexana, KS, USA) for image post-processing and generation of 2D and 3D virtual intravascular endoscopy (VIE) images. VIE visualization provides intraluminal views of the arterial wall and abnormal changes such as stenosis due to calcification, plaque or thrombus, with details of generating VIE views described in our previous studies (26-30). In brief, a CT number thresholding technique was used to generate VIE views of the pulmonary artery and thrombus in these phantom images without being affected by artifact. Selection of an appropriate CT threshold is important to ensure that the VIE images are free from artifact with clear demonstration of intraluminal views of pulmonary artery wall and thrombus. Figure 2 is an example showing the relationship between VIE visualizations and different threshold selections.
Quantitative assessment of image quality
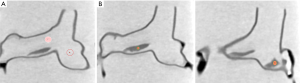
To determine image quality among these CTPA protocols, quantitative assessment of image quality was performed by measuring the image quality in terms of signal-to-noise ratio (SNR) in the main pulmonary arteries and within the thrombus regions. A region of interest (ROI) with an area of 25 mm2 (containing minimum 300 voxels) was placed in the main right and left pulmonary arteries to measure the SNR. In addition, a ROI with an area of 5 mm2 (containing 50 voxels) was placed within the thrombus region to measure SNR among these images. Figure 3 shows measurement of SNR in the main pulmonary arteries and within thrombus regions. Measurements were repeated three times at each location with the mean values used as the final ones to minimize intra-observer variability. All measurements were performed by two observers separately with excellent correlation between the observers (r=0.991, P<0.001) with the mean values used as the final results.
Radiation dose measurement
Volume CT dose index (CTDIvol) and dose length product (DLP) were recorded and compared between these CTPA protocols. Effective dose was calculated using a tissue conversion coefficient of 0.014 mSv/mGy/cm which is commonly used for calculation of chest CT dose (31).
Statistical analysis
Data were entered into SPSS 24.0 (IBM Corporation, Armonk, NY, USA) for statistical analysis. Continuous variables were presented as mean and standard deviation. A paired sample t test was used to determine whether there are any significant differences in SNR measured with different CTPA protocols. A P value of less than 0.05 indicates a statistically significant difference.
Results
CTPA scans were successfully tested on the 3D printed model with use of different imaging protocols. Table 1 shows SNR measurements at images acquired with different CTPA protocols. Apparently, SNR measured within the thrombus on both sides was significantly higher in images acquired with higher kVp such as 100 and 120 protocols than that in the low 70 and 80 kVp protocols (P<0.001). There were no significant differences in SNR measurements across all 100 and 120 kVP protocols (P>0.05), regardless of the pitch values. SNR was significantly lower in the high-pitch protocols with 70 and 80 kVp, when compared to the protocols with use of pitch values of 0.9 and 2.2 (P<0.01).

Full table
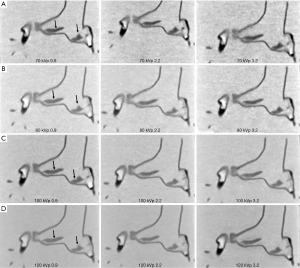
SNR measured outside the thrombus in the main pulmonary arteries did not show any significant differences among these images acquired with 100 and 120 kVp protocols (P>0.05), except for the 120 kVp and pitch 3.2 protocol which shows significantly higher SNR than in the low pitch protocols (P<0.05). Similarly, SNR measured in images (both left and right main pulmonary arteries) acquired with 70 and 80 kVp and pitch of 3.2 protocol was significantly lower than that in other protocols (P<0.05). Figure 4 is an example showing coronal reformatted images of these CTPA protocols. When pitch was increased to 3.2, image noise was increased with use of low kVp protocols such as 70 and 80 kVp as shown in Figure 4A,B. However, the thrombi in the pulmonary arteries are clearly displayed on these images, despite the use of low-dose protocols.
3D VIE images were generated and compared across different CTPA protocols with clear visualization of intraluminal views of pulmonary artery lumen and thrombus. Figure 5 shows a series of VIE images generated with these CTPA protocols. As shown in the images, VIE views of the arterial wall and thrombus were not affected by changing the kVp values, although 100 and 120 protocols produced VIE images with relatively smoother intraluminal appearances (Figure 5A,B,C). VIE images were not affected by changing the pitch from 0.9 to 2.2 (Figure 5A,B), however, when pitch was increased to the high-pitch mode of 3.2, images acquired with the 70 kVp protocol were affected, with irregular appearances of arterial wall and thrombi when compared to those protocols with use of 100 and 120 kVp (Figure 5C).
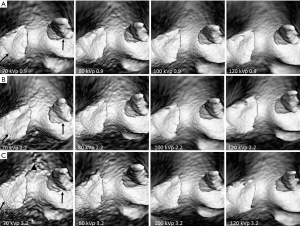
Table 1 shows CTDIvol and DLP as well as effective dose associated with these scanning protocols. With kVp reduced from 120 to 80 and use of high-pitch CTPA protocols, radiation dose was reduced by 33–80% when compared to the low kVp and low pitch protocols without compromising image quality.
Discussion
In this phantom study, we simulated PE in the main pulmonary arteries based on a 3D printed model and tested different CTPA protocols comprising a range of kVp and pitch values. Quantitative assessment of image quality showed no significant differences when kVp was lowered from 120 to 100 or 80 kVp, or pitch was increased from 0.9 to 3.2. This led to a significant dose reduction by more than 80% with use of low-dose CTPA protocols. 3D VIE image visualizations of pulmonary artery and thrombus demonstrated similar findings, although visualization of the thrombus was affected when the high pitch of 3.2 was used in the 70 kVp protocol. This study further confirms the feasibility of using a low-dose CTPA protocol in the detection of PE while maintaining acceptable image quality.
CTPA is currently recommended as the first line imaging modality in the diagnosis of suspected PE, given the high spatial and temporal resolution available with current CT scanners, and the high diagnostic yield (4,13). Technical developments of CT imaging have led to significant dose reductions with use of various dose-saving strategies including low kVp, use of IR algorithms, tube current modulation, high-pitch protocol and use of dual energy CT (2,32,33). Low-dose CTPA using 70 or 80 kVp and high-pitch mode has been proved to achieve diagnostic image quality compared to the standard CTPA protocol, while significantly reducing radiation dose (9,11-14). However, research on the investigation of image quality in normal pulmonary arteries and PE is still limited. This study adds valuable information to the current literature by exploring a variety of CTPA protocols including the lowest kVp and highest pitch value of 3.2 that is available in the literature.
A high-pitch CT protocol is available with fast speed CT scanners and decreases radiation dose significantly when pitch is increased from the standard 0.9 to 2.2 or more than 3.0. However, increasing pitch during CT scans is associated with compromising spatial resolution, which could increase image noise affecting diagnostic quality. We confirmed this in our study as image noise was increased in the 70 and 80 kVp protocols with a pitch of 3.2 (Figure 4A,B). This is especially apparent when visualizing the intraluminal thrombus at the images acquired with 70 kVp and the 3.2 pitch protocol (Figure 5C). Despite this potential limitation, clinical studies have shown the feasibility of using high-pitch CTPA protocols in the diagnosis of PE without losing image quality (12-14,34).
Buchner et al. (34) in their large single center study compared high-pitch CTPA (180 mAs with filtered back projection and pitch 1.2, and 90 mAs with IR, pitch 3.0) with standard pitch (180 mAs and pitch 1.2) and 100 kVp in 382 patients. No significant difference was noticed in image quality among these 3 groups, while significant reduction of radiation dose was found in the high-pitch and low tube current group (P<0.001). Their results are consistent with other reports on the use of combining low kVp with high-pitch protocols (12-15). Lowering kVp to 80 or 70 in the high-pitch CTPA protocol could be challenging due to the potential risk of compromising image quality. This was observed in our study as the SNR measured with 70 and 80 kVp protocols was significantly lower than that measured with 100 or 120 kVp protocols (Table 1). Further, 3D visualization of intraluminal appearances of thrombus and pulmonary artery wall is affected by artifact due to increased image noise with 70 kVp and high pitch 3.2 protocol. Previous studies focused on 2D (axial and multiplanar reformation) images of low-dose CTPA protocols for detection of PE (12-15,34), while in this study, we assessed both 2D and 3D VIE images acquired with different CTPA protocols and corresponding image quality for visualization of PE. Thus, our results provide additional information to the current literature.
With rapid developments in 3D printing techniques and increasing applications in the medical field, patient-specific 3D printed models have been shown to be highly accurate in replicating normal anatomical structures and pathologies (17,20-23). Our recent study (17) has demonstrated the accuracy of a 3D printed pulmonary artery model with successful testing of different CT scanning protocols on the model. To our knowledge, this is the first report of using a patient-specific 3D printed pulmonary model for determining optimal CTPA protocols. Findings of this study are expected to encourage more research 3D printing techniques in other applications to develop low-dose CT protocols.
There are some limitations in this study. First, despite a realistic 3D printed model being used for studying different CTPA protocols, the model was not placed in an environment which simulated normal anatomic regions such as lungs, ribs, bones or heart. Thus, the radiation dose associated with these protocols is much lower than the actual value as reported in other studies due to the small field of view in these CT scans. Confirmation of results with simulation of normal thoracic structures are required. Second, only SNR was measured to determine image quality while no contrast-to-noise ratio (CNR) was measured. This is due to the reason that the 3D printed model was immersed in the diluted contrast medium instead of only filling the pulmonary arteries with contrast medium. Quantitative assessment of image quality by using both SNR and CNR would allow us to draw robust conclusions. Third, PE was simulated in the main pulmonary arteries, while no blood clot was used in the peripheral arteries to simulate embolism. Although CTPA has high diagnostic value, accurate detection of peripheral (segmental or subsegmental) or small thrombus in the peripheral pulmonary artery branches with a low-dose protocol would be required. This is currently being investigated with the aim of simulating small emboli in the peripheral arterial branches. Finally, no subjective assessment of image quality was included due to the fact that the pulmonary emboli were large. This could be assessed in the ongoing study with simulation of peripheral PE with different CTPA protocols.
In conclusion, we have demonstrated the feasibility of simulating PE in a 3D printed pulmonary model with different CT scanning protocols tested. Low-dose CTPA protocol is achievable with use of low kVp such as 70 or 80 with acceptable image quality. When high-pitch of 3.2 is used for CTPA, kVp can be lowered to 80 or 100 without compromising image quality in most of the protocols. Use of a low-dose CTPA protocol by combining 70 kVp with high-pitch 3.2 should be avoided due to its negative impact on the image quality of both pulmonary arteries and thrombus, as well as on intraluminal visualization of thrombus and pulmonary artery wall. Further studies on a low-dose CTPA protocols for detection of peripheral PE are underway.
Acknowledgments
Authors would like to thank Mr Tom Tiang from Perth Children’s Hospital for his assistance in CT scanning of the pulmonary artery model.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: No ethical approval is required since this study is based on a phantom experiment.
References
- Sherk WM, Stojanovska J. Role of clinical decision tools in the diagnosis of pulmonary embolism. AJR Am J Roentgenol 2017;208:W60-70. [Crossref] [PubMed]
- Albrecht MH, Bickford MW, Nance JW Jr, Zhang L, De Cecco CN, Wichmann JL, Vogl TJ, Schoepf UJ. State-of-the-Art pulmonary CT angiography for acute pulmonary embolism. AJR Am J Roentgenol 2017;208:495-504. [Crossref] [PubMed]
- Kligerman SJ, Mitchell JW, Sechrist JW, Meeks AK, Galvin JR, White CS. Radiologist performance in the detection of pulmonary embolism: Features that favor correct interpretation and risk factors for errors. J Thorac Imaging 2018;33:350-7. [PubMed]
- Sun Z, Lei J. Diagnostic yield of CT pulmonary angiography in the diagnosis of pulmonary embolism: a single center experience. Interv Cardiol 2017;9:191-8. [Crossref]
- Ong CW, Malipatil V, Lavercombe M, Teo MG, Coughlin PB, Leach D, Spanger MC, Thien F. Implementation of a clinical prediction tool for pulmonary embolism diagnosis in a tertiary teaching hospital reduces the number of computed tomography pulmonary angiograms performed. Intern Med J 2013;43:169-74. [Crossref] [PubMed]
- Chen EL, Ross JA, Grant C, Wilbur A, Mehta N, Hart E, Mar WA. Improved image quality of low-dose CT pulmonary angiograms. J Am Coll Radiol 2017;14:648-53. [Crossref] [PubMed]
- Wichmann JL, Hu X, Kerl JM, Schulz B, Frellesen C, Bodelle B, Kaup M, Scholtz JE, Lehnert T, Vogl TJ, Bauer RW. 70 kVp computed tomography pulmonary angiography: potential for reduction of iodine load and radiation dose. J Thorac Imaging 2015;30:69-76. [Crossref] [PubMed]
- Martini K, Meier A, Higashhigaito K, Saltybaeva N, Alkadhi H, Frauenfelder T. Prospective randomized comparison of high-pitch CT at 80 kVp under free breathing with standard-pitch CT at 100 kVp under breath-hold for detection of pulmonary embolism. Acad Radiol 2016;23:1335-41. [Crossref] [PubMed]
- Li X, Ni QQ, Schoepf UJ, Wichmann JL, Felmly LM, Qi L, Kong X, Zhou CS, Luo S, Zhang LJ, Lu GM. 70-kVp high-pitch computed tomography pulmonary angiography with 40 mL contrast agent: initial experience. Acad Radiol 2015;22:1562-70. [Crossref] [PubMed]
- Kligerman S, Lahiji K, Weihe E, Lin CT, Terpenning S, Jeudy J, Frazier A, Pugatch R, Galvin JR, Mittal D, Kothari K, White CS. Detection of pulmonary embolism on computed tomography: improvement using a model-based iterative reconstruction algorithm compared with filtered back projection and iterative reconstruction algorithms. J Thorac Imaging 2015;30:60-8. [Crossref] [PubMed]
- Bolen MA, Renapurkar RD, Popovic ZB, Popovic ZB, Heresi GA, Flamm SD, Lau CT, Lau CT, Halliburton SS. High-pitch ECG synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013;201:971-6. [Crossref] [PubMed]
- Lu GM, Luo S, Meinel FG, McQuiston AD, Zhou CS, Kong X, Zhao YE, Zheng L, Schoepf UJ, Zhang LJ. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol 2014;24:3260-8. [Crossref] [PubMed]
- Sabel BO, Buric K, Karara N, Thierfelder KM, Dinkel J, Sommer WH, Meinel FG. High-pitch CT pulmonary angiography in third generation dual-source CT: image quality in an unselected patient population. PLoS ONE 2016;11:e0146949. [Crossref] [PubMed]
- Boos J, Kropil P, Lanzman RS, Aissa J, Schleich C, Heusch P, Sawichi LM, Antoch G, Thomas C. CT pulmonary angiography: simultaneous low-pitch dual-source acquisition mode with 70 kVp and 40 ml of contrast medium and comparison with high-pitch spiral dual-source acquisition with automated tube potential selection. Br J Radiol 2016;89:20151059. [Crossref] [PubMed]
- Laqmani A, Regier M, Veldhoen S, Backhaus A, Wassenberg F, Sehner S, Groth M, Nagel HD, Adam G, Henes FO. Improved image quality and low radiation dose with hybrid iterative reconstruction with 80 kV CT pulmonary angiography. Eur J Radiol 2014;83:1962-9. [Crossref] [PubMed]
- Hu X, Ma L, Zhang J, Li Z, Shen Y, Hu D. Use of pulmonary CT angiography with low tube voltage and low-iodine-concentration contrast agent to diagnose pulmonary embolism. Sci Rep 2017;7:12741. [Crossref] [PubMed]
- Aldosari S, Squelch A, Sun Z. Patient-specific 3D printed pulmonary artery model: A preliminary study. Digit Med 2017;3:170-7. [Crossref]
- Olivieri LJ, Krieger A, Loke YH, Nath DS, Kim PC, Sable CA. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. J Am Soc Echocardiogr 2015;28:392-97. [Crossref] [PubMed]
- Cantinotti M, Valverde I, Kutty S. Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging 2017;33:137-44. [Crossref] [PubMed]
- Lau IW, Liu D, Xu L, Fan Z, Sun Z. Clinical value of patient-specific three-dimensional printing of congenital heart disease: Quantitative and qualitative Assessments. PLoS One 2018;13:e0194333. [Crossref] [PubMed]
- Liu D, Sun Z, Chaichana T, Ducke W, Fan Z. Patient-specific 3D printed models of renal tumours using home-made 3D printer in comparison with commercial 3D printer. J Med Imaging Health Inf 2018;8:303-8. [Crossref]
- Sun Z, Liu D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant Imaging Med Surg 2018;8:311-25. [Crossref] [PubMed]
- Lau I, Sun Z. Three-dimensional printing in congenital heart disease: A systematic review. J Med Radiat Sci 2018;65:226-36. [Crossref] [PubMed]
- Shapeways. Frequently Asked Questions. Available online: https://www.shapeways.com/support/faq
- 3D Printing Materials. Shapeways. Available online: https://www.shapeways.com/materials/
- Xu L, Sun Z. Virtual intravascular endoscopy visualization of calcified coronary plaques: a novel approach of identifying plaque features for more accurate assessment of coronary lumen stenosis. Medicine 2015;94:e805. [Crossref] [PubMed]
- Sun Z, Dosari SA, Ng C, al-Muntashari A, Almaliky S. Multislice CT virtual intravascular endoscopy for assessing pulmonary embolisms: a pictorial review. Korean J Radiol 2010;11:222-30. [Crossref] [PubMed]
- Sun Z, Dimpudus FJ, Nugroho J, Adipranoto JD. CT virtual intravascular endoscopy assessment of coronary artery plaques: a preliminary study. Eur J Radiol 2010;75:e112-9. [Crossref] [PubMed]
- Sun Z, Winder JR, Kelly BE, Ellis PK, Kennedy PT, Hirst DG. Assessment of VIE image quality using helical CT angiography: in vitro phantom study. Comput Med Imaging Graph 2004;28:3-12. [Crossref] [PubMed]
- Sun Z, Gallagher E. Multislice CT virtual intravascular endoscopy for abdominal aortic aneurysm stent grafts. J Vasc Interv Radiol 2004;15:961-70. [Crossref] [PubMed]
- McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am 2009;47:27-40. [Crossref] [PubMed]
- Henzler T, Barraza JM, Nance JW Jr, Costello P, Krissak R, Fink C, Schoepf UJ. CT imaging of acute pulmonary embolism. J Cardiovasc Comput Tomogr 2011;5:3-11. [Crossref] [PubMed]
- Zhang LJ, Lu GM, Meinel FG, McQuiston AD, Ravenel JG, Schoepf UJ. Computed tomography of acute pulmonary embolism: state-of-the-art. Eur Radiol 2015;25:2547-57. [Crossref] [PubMed]
- Bucher AM, Kerl MJ, Albrecht MH, Beeres M, Ackermann H, Wichmann JL, Vogl TJ, Bauer RW, Lehnert T. Systematic comparison of reduced tube current protocols for high-pitch and standard-pitch pulmonary CT angiography in a large single-center population. Acad Radiol 2016;23:619-27. [Crossref] [PubMed]


