Responsive alginate-cisplatin nanogels for selective imaging and combined chemo/radio therapy of proliferating macrophages
Introduction
Atherosclerosis is a disease where lipids and fibrous elements accumulate in arteries and is the underlying cause of more than 50% of deaths in the westernized world (1). The early stages of atherosclerosis are marked by subendothelial accumulation of cholesterol-engorged macrophages, later joined by smooth muscle cells and necrotic debris leading to plaque formation (1,2). As macrophages play a pivotal role in development of atherosclerosis as well as atheroma plaque destabilization and rupture, which in turn leads frequently to thrombo-occlusive complications including strokes and myocardial infarction, macrophages may be a valuable therapeutic target. Indeed, previous studies support targeting macrophages as a promising strategy for atherosclerosis treatment (2).
Recent studies have shown that the majority of macrophages in atherosclerotic lesions originate from local proliferation rather than inflammatory monocyte recruitment from the bloodstream (3). This suggests that use of antiproliferation agents may be an effective and novel approach to treat atherosclerosis. Antitumor drugs such as doxorubicin (4) and photodynamic therapy (5) are examples of antiproliferation treatments. However, development of theranostic agents which can selectively target and treat proliferating macrophages in atherosclerotic regions is an ongoing challenge.
Cisplatin is a widely used platinum-based drug included in many standard regimens for cancer treatment, sometimes in combination with radiotherapy. Cisplatin acts on cells by crosslinking DNA, resulting in DNA damage. Cisplatin also has radiosensitizing properties. The tendency of cisplatin to form crosslinks with carboxyl or sulfhydryl groups in proteins or specific nitrogen atoms in DNA has led to use in incorporation in polymers (6) and other carriers to overcome drug resistance and drug side effects such as nephrotoxicity (7,8). Alginic acid is a polymer obtained from seaweed known for being inexpensive, nontoxic, and biocompatible (9). Alginate gelates in the presence of cations such as Ca2+ (9). However, we demonstrated that it does not readily gelate with Pt2+ for nanogel synthesis.
In this study, we synthesized theranostic alginate-based nanogels (TANgel) as a pH-responsive drug-releasing theranostic nanoplatform for macrophage cells (Figure 1). Initially, carboxylic acid groups of alginate were modified with iminodiacetic acid (IDA) to enhance chelation with platinum ions (10,11). Near infrared (NIR) fluorophore ATTO655 was conjugated to the modified alginic acid. Then, cisplatin, which functions as an antiproliferation drug, was used to crosslink alginate molecules to form TANgel (Figure 1A). This was the first synthesis of TANgel. We hypothesized that TANgel would be stable in blood circulation, minimizing systemic toxicity of cisplatin. As atherosclerotic plaques have leaky vascular structure resulting in enhanced permeability (12,13), TANgel can accumulate in leaky regions and be actively taken up by macrophage cells. In macrophages, release of cisplatin may be triggered at lysosomal pH (pH 4.5–5.5) because of pH-dependent decreased affinity of IDA (as well as carboxylic acids of alginic acid) for metal ions (14). The sites at which this occurs would then be detected through NIR fluorescence imaging. In addition, combined treatment with radiation therapy (RT) may further reduce the therapeutic concentration of the drug with regard to reducing macrophage proliferation (Figure 1B). Therefore, TANgel may be useful for selective imaging and combined chemo/radio therapy of proliferating macrophage cells in atherosclerotic regions with reduced systemic toxicity.
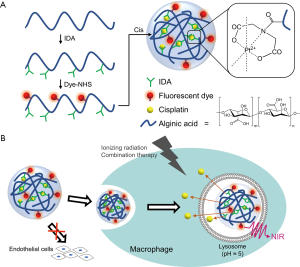
Methods
Materials
IDA, sodium alginate (MW =190 kDa), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), ethylenediamine, ATTO655-NHS ester, cisplatin [cis-diamminedichloridoplatinum (II)], and o-phenylenediamine (OPDA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dialysis membrane (MWCO =10 kDa) was purchased from Repligen (Waltham, MA, USA) and dialysis tubing (MWCO =12 kDa) was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Synthesis of IDA-modified alginic acid (Alg-IDA)
IDA was attached to the alginic acid backbone by utilizing EDC. Sodium alginate (200 mg) was dissolved in 10 mL of deionized (DI) water. Five milliliters of EDC aqueous solution (200 mM) was added and the mixture was adjusted to pH 6 by addition of 0.1 M hydrochloric acid. After stirring for 45 min, IDA (200 mg) was dissolved in 4 mL of sodium hydroxide solution (0.1 M), and the solution was added to the mixture in a dropwise manner. The mixture was re-adjusted to pH 6 and stirred for 3 h. Following stirring, the mixture was dialyzed (MWCO =15 kDa) in 1 mM hydrochloric acid for 2 days, in 1 mM hydrochloric acid +1% sodium chloride solution for 1 day, then in DI water for 1 day. Finally, the product was freeze-dried and stored. To synthesize the Alg-IDA-dye conjugate, one-tenth of the IDA solution was combined with equimolar ethylenediamine (11 mg). Two and one-half milliliters of the resulting product was concentrated to a volume of 0.5 mL using a centrifugal filter, to which 2 mL phosphate buffer (pH 8.5) and 1 µmol ATTO655-NHS ester was added. The mixture was shaken for 1 h, then dialyzed (MWCO =10 kDa) in DI water for 2 days. The product was freeze-dried for storage.
Preparation of TANgel
Cisplatin was dissolved in DI water at 5 mg/mL and preheated to 90 °C for dissolution. Alg-IDA and Alg-IDA-dye, at a mass ratio of 9:1, were dissolved in water to a final concentration of 2 mg/mL. Then 0.2 mL of cisplatin solution was mixed with 0.8 mL of Alg-IDA/Alg-IDA-dye solution, and the mixture was incubated at 95 °C for 1 h. Following incubation, the mixture was cooled on ice for 15 min. The mixture was then dialyzed (MWCO =2 kDa) in DI water for 4 h.
Characterizations
UV-Vis absorption spectra of free dye, Alg-IDA-dye, and TANgel were obtained using a UV-Vis spectrophotometer DU730 (Beckman Coulter, USA). Fluorescence spectra of the solutions (λex =655 and 710 nm) were obtained on a multifunctional microplate reader (Safire 2, Tecan, Switzerland). Scanning electron microscope (SEM) images of nanoparticles were obtained using a JEM-7800F field emission SEM (JEOL, Japan). EDS data was obtained using an X-max (Oxford Instruments, UK) analyzer attached to a JEM-F200 (JEOL) transmission electronic microscope (TEM) in scanning TEM mode. Dynamic light scattering (DLS) and zeta potential analysis were performed on a Zetasizer Nano-ZS (Malvern Instruments, UK). Inductively coupled plasma mass spectrometry (ICP-MS) measurement was performed on a NexION 300 (PerkinElmer, USA). Cisplatin equivalent concentration was determined using ICP-MS results and the freeze-dried mass of TANgel. Fluorescence photographs were taken with in vitro imaging system (IVIS) Lumina XR (Caliper Life Science, USA).
In vitro drug release test
Release of cisplatin from TANgel was analyzed at different pH conditions. TANgel solution (625 µg/250 µL) was transferred to dialysis microtubes (MWCO =12 kDa) and immersed in 40 mL of phosphate buffer solutions (0.1 M) at pH 7.4 or pH 5 and shaken gently. At each time point 1.5 mL of buffer solution was collected and 1.5 mL of fresh buffer was added back. The amount of cisplatin in each sample was determined with a modified version of the OPDA assay (15). Total cisplatin released was calculated cumulatively. Experiments were performed in triplicate.
Cell culture
A Murine macrophage cell line (J774A.1), a human primary dermal microvascular endothelial cell line (HDMVECn), a human primary dermal fibroblast cell line (HDF), and a human primary coronary artery smooth muscle cell line (HCASMC) were acquired from ATCC (American Type Culture Collection, Manassas, VA, USA). J774A.1 and HDF cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic and antimycotic (Thermo Fisher Scientific). HDMVECn and HCASMC cells were maintained in vascular cell basal medium plus microvascular endothelial growth kit (ATCC). Cells were incubated in a humidified incubation chamber containing 5% CO2.
Cell uptake assay
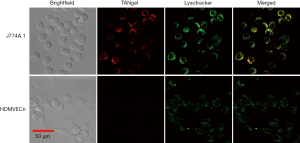
J774A.1 and HDMVECn cells were plated in an 8-well Lab-Tek II chambered glass (Thermo Fisher Scientific) at a density of 5×104 cells/well, and incubated for 24 h to allow cell attachment. TANgel solution was diluted with the appropriate cell culture medium to achieve a concentration of 2 µM Cis equivalent. The existing culture medium was replaced with 200 µL of fresh medium containing TANgel, and cells were incubated for 24 h. Untreated control cells were incubated with cell culture medium without TANgel for 24 h. All cells were washed 3 times with fresh culture medium and stained with 50 nM Lysotracker Red DND-99 (Thermo Fisher Scientific). Fluorescence images of the cells (λex =633 nm, λem =652–747 nm for TANgel; λex =561 nm, λem =579–633 nm for Lysotracker) were obtained using confocal laser scanning microscopy (LSM 780, Carl Zeiss, Germany). All fluorescence images for TANgel were taken using identical settings for comparison.
Cytotoxicity assay
J774A.1 cells were seeded in a 96-well plate at a density of 5×103 cells/well. HDMVECn, HDF and HCASMC cells were seeded in a 96-well plate at a density of 1×104 cells/well. All cells were incubated for 24 h to allow cell attachment. TANgel and free Cis were diluted in cell culture medium to obtain equivalent concentrations of 0.1, 0.2, 0.5, 1 and 2 µM Cis for J774A.1 experiments, and concentrations of 1 and 2.5 µM Cis for HDMVECn, HDF, and HCASMC experiments. The existing culture medium was replaced with 100 µL of the appropriate media. Cells were incubated for 24 h then washed and the media in each well exchanged with fresh drug-free cell culture medium. To evaluate the effect of TANgel on chemo/radio therapy, J774A.1 cells were treated with TANgel or free Cis in DMEM described above for 24 h. Following treatment, the culture media were exchanged with fresh drug-free media. Cells were then irradiated with ionizing radiation. Radiation treatments were performed using a XenX irradiator platform (Xstrahl, UK) to deliver a total X-ray dose of 3 Gy.
Following radiation, cells were incubated for 72 h, and cell viability was analyzed using a CCK-8 assay kit (Dojindo Laboratories, Japan). Absorbance of each sample was measured at 450 nm using a microplate reader. Untreated control cells were used as a reference for 100% viable cells, and their medium served as the background. Data are expressed as mean ± SD of 6 data samples.
Live cell proliferation assay
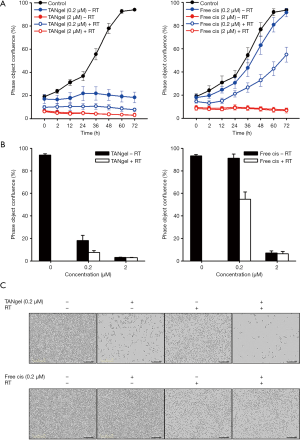
The effect of TANgel or free Cis with or without radiation treatment on the rate of cell proliferation was evaluated. Cells were seeded in 96-well plates at a density of 5,000 cells/well and incubated overnight for cell attachment. Cells were treated with TANgel or free Cis (0.2 or 2 µM, respectively) for 24 h. Culture media was replaced, and cells were irradiated with ionizing radiation and were analyzed every 2 h for 72 h using an IncuCyte ZOOM live cell analysis device (Essen BioScience, Ann Arbor, MI, USA). All experiments were carried out in triplicate. Data are expressed as mean ± SD of 4 data samples.
Statistical analysis
Statistical analysis was performed using Student’s t-test.
Results
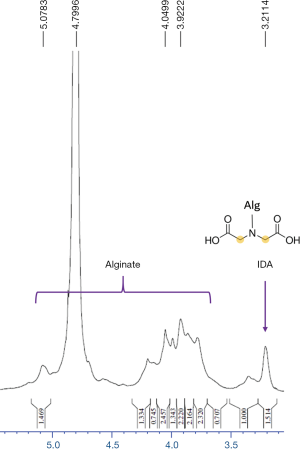
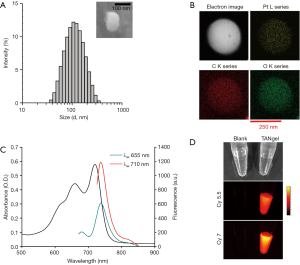
To enhance cisplatin-binding performance of the alginic acid backbone, we attached IDA to carboxylic acids of alginate using carbodiimide. Success of the reaction was confirmed by identification of Alg-IDA by 1H-NMR (Figure S1). To allow for imaging, Alg-IDA was further functionalized with amine groups via ethylenediamine, to which fluorescent dye molecules were attached by NHS ester reaction. After obtaining fluorescent alginate molecules (Alg-IDA-dye), nanogel synthesis was carried out by mixing cisplatin with Alg-IDA and Alg-IDA-dye, and heating the mixture at 95 °C for 1 h. The optimal ratio and concentrations of Alg-IDA species and cisplatin were determined in a preliminary experiment (data not shown). After formation, TANgel was characterized through multiple means. DLS measurements gave hydrodynamic sizes of 99.3 nm with a polydispersity index (PDI) of 0.177 and zeta potential of −28.8 mV (Figure 2A). To determine cisplatin loading, TANgel was evaluated by ICP-MS, with a result of 8.96±0.21 wt% of Pt content, which translates to a cisplatin loading efficiency of (13.78±0.32)%. Note that the Pt content agrees with the value obtained from energy-dispersive X-ray spectroscopy (EDS) which gave 13.99 wt% of Pt. EDS mapping also showed Pt atoms evenly distributed throughout the nanogel particle (Figure 2B). In UV-Vis absorbance and fluorescence spectra, new absorption and emission peaks emerged in the region of 710 and 740 nm, respectively (Figure 2C). NIR fluorescence imaging showed that the fluorescence from TANgel could be detected through a longer-wavelength channel where free dye molecules showed no emission (Figure 2D, Figure S2).
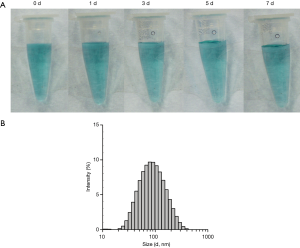
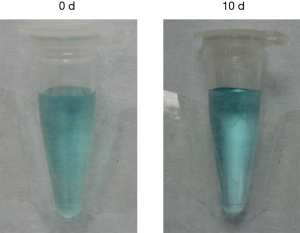
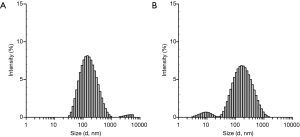
Analysis of dispersion stability of TANgel (Figure S3) showed that the solution remained stable for at least 7 days under ambient conditions. DLS measurement of the sample at day 7 showed no notable changes to the hydrodynamic size distribution of the nanogels (Figure S3B). Likewise, nanogels suspended in physiological solution were stable for at least 10 days (Figure S4). We also evaluated the effect of exchanging medium with buffer solutions at pH 7.4 and pH 5, and demonstrated little change in size distribution of TANgel after 48 h (Figure S5).
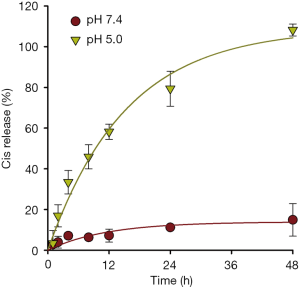
Drug release profile was determined by placing TANgel solutions in dialysis tubes and shaking in phosphate buffer solutions at pH 7.4 or pH 5 (Figure 3). Cisplatin released much faster at pH 5, as expected, with all incorporated cisplatin released within 48 h. At pH 7.4, less than 15% was released within 48 h.
We evaluated intracellular uptake of TANgel and its localization in vitro. As atherosclerosis is characterized by plaque build-up and macrophage accumulation in arteries, the J774A.1 macrophage cell line was chosen to model atherosclerosis. To model normal cells the HDMVECn human vascular endothelial cell line was used. Both cell types were treated with TANgel for 24 h. Cells were washed and NIR fluorescence images obtained (Figure 4). Although J774A.1 cells clearly displayed fluorescence signals from TANgel, HDMVECn cells did not display any significant fluorescence from TANgel. Also, in J774A.1 cells TANgel fluorescence was mainly coincident with fluorescence from Lysotracker, indicating that when TANgel is taken up by macrophage cells they are localized in lysosomes. As lysosomes have an acidic environment, TANgel can be expected to release cisplatin reliably because of its pH-responsiveness.
To test the potential of combination therapy on macrophage cells, in vitro toxicity was assessed (Figure 5). J774A.1 cells were treated with free cisplatin or TANgel over a concentration range of 0.1–2 µM. Half of the cells were subjected to RT (3 Gy). Cell viability was assayed after 3 days to evaluate DNA damage resulting from radiation and chemotherapy. Results showed that while TANgel showed similar toxicity in concentrations of 0.5 µM and above, at lower concentrations it was more effective at inhibiting cell viability, both with and without RT. Indeed, the IC50 of TANgel was calculated at 0.12 µM (cisplatin equivalent), lower than free cisplatin, which had an IC50 of 0.21 µM. This difference may be explained by the high uptake rate of TANgel and polymer ligands serving as protection against platinum drug detoxification by thiol residues and other chemical reactions. As a measure to increase the reliability of cytotoxicity data, J774A.1 cells were treated in a live cell analysis system (Figure 6). The results were similar to those seen using the more traditional assay above, including the result demonstrating higher treatment efficiency of TANgel at 0.2 µM.
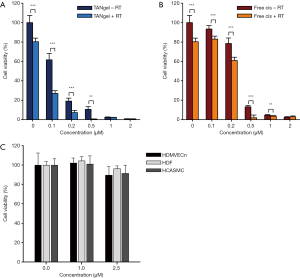
To test selectivity of TANgel treatment, normal cell lines were treated with TANgel over a concentration range of 0–2.5 µM. In addition to HDMVECn, two different suitable primary human cell lines were used: HDF dermal fibroblasts and HCASMC coronary artery smooth muscle cells. Results showed no toxicity at 1 and 2.5 µM as the difference in cell viability compared to the control group was not statistically significant (P=0.128, 0.171, and 0.157, respectively). This result, combined with the selectivity of radiotherapy, signifies that collateral damage to other normal tissues can be minimized, during treatment with TANgel.
Discussion
Macrophages play a pivotal role in development of atherosclerosis. Proteases secreted from macrophages degrade the extracellular matrix in the lesions, leading to plaque rupture which can cause thromboembolic stroke or myocardial infarction. These characteristics highlight why development of theranostic agents which can selectively image and treat proliferating macrophages in the atherosclerotic region has great therapeutic potential.
We proposed cisplatin-loaded TANgel as a pH-responsive drug-releasing nanotheranostic for NIR fluorescence imaging and combined chemo/radio therapy of proliferating macrophages. Cisplatin was selected as an antiproliferation drug to treat macrophages and as a crosslinking agent of polymer backbones, enabling simple preparation of nanogels. As shown in Figure 2A and Figure S3, spherical shaped nanogels with a hydrodynamic size of 99.3 nm were formed, and TANgel was well dispersed in aqueous solutions without significant changes in hydrodynamic size for at least 7 d. This result suggests that cisplatin crosslinked efficiently, supporting the three-dimensional structure of TANgel.
As the alginate backbone is rich in carboxylic acid groups, alginate-based nanogels are likely to be pH-responsive. In a recent example, a doxorubicin-loaded alginate nanogel was prepared by mixing doxorubicin with alginate to take advantage of the electrostatic interaction between the molecules, followed by cross-linking with calcium ions. The release rate of doxorubicin from the nanogel was faster at pH 5.0 than at pH 7.4; however, a sizable amount of drug (~40%) was released after 24 h at pH 7.4 (16). In the current study, we showed that approximately 80% of incorporated cisplatin was released from TANgel at pH 5 within 24 h, while less than 12% was released at pH 7.4 within 24 h. We hypothesize that this results from use of an additional chelating moiety with pH-sensitivity and use of platinum ions in lieu of calcium ions. The difference in pH sensitivity supported the hypothesis that cisplatin will be preferentially released from TANgel in the acidic environment of intracellular lysosomes, but not at normal physiological pH (Figure 1B). Indeed, good therapeutic efficacy was obtained in TANgel-treated macrophages while no cytotoxicity was observed with up to 2.5 µM cisplatin equivalent treatment of various normal cells. This shows the potential utility of TANgel as a highly efficient pH-responsive drug delivery system for treatment of disease.
Recently, NIR fluorescence imaging has emerged as a promising technique for real-time visualization of sentinel lymph nodes, tumor tissue, and vital structures during intraoperative procedures, allowing for accurate guidance during surgery. Interestingly, the conjugated NIR dye ATTO655 in TANgel has additional excitation and emission wavelengths at a much longer NIR wavelength range compared to free ATTO655 dye. We speculate that this is due to J-aggregation of dye molecules inside TANgel (17). This shift in both the peak absorption and emission wavelength allowed us to take fluorescence images in the longer NIR wavelength region, where TANgel exhibited strong emission but the original dye molecules did not (Figure 2D and Figure S2). As TANgel was preferentially taken up by macrophage cells compared to normal vascular endothelial cells (Figure 4), TANgel may be useful in selective NIR fluorescence imaging of macrophage cells in atherosclerotic lesions.
In this article, we presented a theranostic nanoplatform in which the crosslinker doubles as a chemotherapy drug and a radiosensitizer. Synthesis of this theranostic nanoplatform was accomplished using a dramatically simplified novel approach. Synthesis of this nanogel is simple and proceeds under mild conditions. Our product, TANgel, was shown to have a good size distribution, stability, excellent pH-responsiveness, selective uptake, and interesting imaging properties owing to the shift in peak wavelengths. Simple components, practically consisting of only alginate derivative and cisplatin, would be beneficial for clinical use. In the future, additional drugs can be loaded onto this nanogel to target other ailments.
Acknowledgements
Funding: This work was supported by the National Research Foundation of Korea (NRF) (grants NRF-2015M2A2A6A01044298), and also from the project titled “Development of marine material based near infrared fluorophore complex and diagnostic imaging instruments [2018]” funded by the Ministry of Oceans and Fisheries, Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lusis AJ. Atherosclerosis. Nature 2000;407:233-41. [Crossref] [PubMed]
- Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol 2009;7:234-43. [Crossref] [PubMed]
- Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166-72. [Crossref] [PubMed]
- Park D, Cho Y, Goh SH, Choi Y. Hyaluronic acid-polypyrrole nanoparticles as pH-responsive theranostics. Chem Commun (Camb) 2014;50:15014-7. [Crossref] [PubMed]
- Kim H, Kim Y, Kim IH, Kim K, Choi Y. ROS-responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics 2013;4:1-11. [Crossref] [PubMed]
- Zhang W, Tung CH. Cisplatin Cross-Linked Multifunctional Nanodrugplexes for Combination Therapy. ACS Appl Mater Interfaces 2017;9:8547-55. [Crossref] [PubMed]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 2008;73:994-1007. [Crossref] [PubMed]
- Pa Ma, Xiao H, Li C, Dai Y, Cheng Z, Hou Z, Lin J. Inorganic nanocarriers for platinum drug delivery. Mater Today 2015;18:554-64. [Crossref]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106-26. [Crossref] [PubMed]
- Yuchi A, Sato T, Morimoto Y, Mizuno H, Wada H. Adsorption mechanism of trivalent metal ions on chelating resins containing iminodiacetic Acid groups with reference to selectivity. Anal Chem 1997;69:2941-4. [Crossref] [PubMed]
- Ohta S, Hiramoto S, Amano Y, Sato M, Suzuki Y, Shinohara M, Emoto S, Yamaguchi H, Ishigami H, Sakai Y, Kitayama J, Ito T. Production of Cisplatin-Incorporating Hyaluronan Nanogels via Chelating Ligand-Metal Coordination. Bioconjug Chem 2016;27:504-8. [Crossref] [PubMed]
- Mause SF, Weber C. Intrusion through the fragile back door: immature plaque microvessels as entry portals for leukocytes and erythrocytes in atherosclerosis. J Am Coll Cardiol 2009;53:1528-31. [Crossref] [PubMed]
- Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov 2011;10:835-52. [Crossref] [PubMed]
- Chaberek S Jr, Martell AE. Stability of metal chelates. I. iminodiacetic and iminodipropionic acids. J Am Chem Soc 1952;74:5052-6. [Crossref]
- Basotra M, Singh SK, Gulati M. Development and validation of a simple and sensitive spectrometric method for estimation of cisplatin hydrochloride in tablet dosage forms: application to dissolution studies. ISRN Anal Chem 2013;2013. [Crossref]
- Xue Y, Xia X, Yu B, Luo X, Cai N, Long S, Yu F. A green and facile method for the preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin. RSC Adv 2015;5:73416-23. [Crossref]
- Würthner F, Kaiser TE, Saha-Möller CR. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew Chem Int Ed Engl 2011;50:3376-410. [Crossref] [PubMed]


