The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications
Introduction
Lung cancer has been one of the most common cancers for several decades, with an estimated number of 1.8 million new cases in 2012 (1). It is the second most common cancer in men after prostate cancer, and the second most common cancer in women, closely following breast cancer. In the UK, between 2003–2005 and 2012–2014, the overall lung cancer incidence rate has increased by 4%, with a striking 18% increase in females. This reflects the changing prevalence of risk factors, specifically the use of tobacco (2). The accurate staging of lung cancer is a crucial part in the management of these patients, as staging of the lung cancer at time of initial diagnosis is the most important predictor of survival. Moreover, the treatment options vary depending on disease stage (3). The TNM staging as we know today, was conceptualized by Pierre Denoix between 1943 and 1952 (4). In 1953, this was accepted by the Union for International Cancer Control (UICC) Committee on Tumour Nomenclature and Statistics, as the basis for anatomical staging. Moving forward to 1996, there was realization for the need to develop an international database for external validity in future TNM editions. The International Association for the Study of Lung Cancer (IASCL), being the only global organization dedicated to the study of lung cancer, with representatives from all disciplines involved in lung cancer care, was best suited to take on this project. This includes the collection and analysis of lung cancer data, and the recommendation of TNM staging updates (5). The project is performed with biostatistical partners at Cancer Research and Biostatistics (CRAB) (3).
Since the establishment of the International Staging Committee in 1997, collecting data on lung cancer cases treated by all modalities from across the globe, this project has now collected over 200,000 cases, and is involved in the two later revisions of the TNM lung cancer staging. IASCL has been accepted as the primary source of recommendations by the UICC for lung cancer staging (4). The 7th edition of the TNM staging was published in 2009, based on retrospective data of 81,496 patients (5). There were limitations in which the original datasets were not designed for TNM staging, and not all the descriptors were validated (6). Also, it was felt that there was inadequate worldwide representation. The data for the 7th edition was also collected in the ‘90s, when the use of positron emission tomography (PET) for staging was not readily available. These limitations prompted the IASLC to launch a new round of data collection in preparation for a new, or the 8th, edition (6). The data collection was also expanded to incorporate mesothelioma, thymic malignancy as well as oesophageal cancer, which will not be covered in this article.
8th edition TNM classification
The database for the 8th edition was collected between 1999 and 2010, from 16 different countries of 35 sources. As advised by Cancer Research and Biostatistics (CRAB), the data for the 8th edition were kept separate from the data accrued for the earlier edition (4). 94,708 cases were assessed, and 77,156 patients finally included in the study. The 17,552 patients were excluded mainly because of unknown or different histology, as well as incomplete stage information (3). Of these patients, 70,967 were comprised of non-small cell lung cancer (NSCLC), and the 6,189, small cell lung cancer (SCLC). The number of NSCLC is similar as the 7th edition, whilst there is a reduction of SCLC by 50% since the previous edition. A total of 73,251 cases were retrospectively registered for T and N descriptors. As for the analysis of the M group, 3,905 cases were prospectively collected through the online electronic data capture system (3).
In addition to the different descriptors used in the earlier edition, this data is further finessed to include 23 non-anatomical elements in the data dictionary for the development of prognostic groups. This included patient-related elements such as demographics and smoking history; tumour-related factors such as the maximum standard uptake value on PET (SUV max) for T and N, grade of tumour; as well as environmentally-related factors such as method of cancer detection and geographical information (3). In terms of the geographical distribution of the data collected, Europe is the leading contributing region, making up 49% of the cases contributed. There is more data from Asia (44%, previously 21%), particularly from Japan, South Korea and China. However, there is less representation from North America (5%, previously 21%) and Australia (1.7%, dropped from 9.3%) (3). In terms of treatment, as in the previous database, patients undergoing surgical treatment alone are predominant, with nearly 85% underwent surgical treatment, either alone, or in combination with chemoradiotherapy (3). The TNM 8th staging was released in 2017. The major highlight of the 8th edition is in the sub- and re-classification of the different stages of lung cancer, based on prognostic data. The main changes are related to the tumour size, extent of involvement (both within the T stage), and the subclassification of the extrathoracic metastases (M stage). These are as outlined below.
Tumour staging (T)
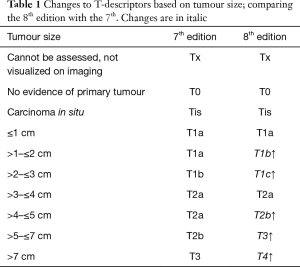
T stage is determined by the size of the primary tumour in long axis measured in multiplanar reconstruction, and its involvement with the adjacent structure/s (7). For T-stage, prognosis was analysed in patients with NSCLC, with or without nodal involvement, and without metastatic disease. The patients who were pathologically staged were analysed separately to those who were clinically staged. A total of 30,102 clinically-staged patients were analysed (8). The findings are consistent in the two groups, in which there is a poorer prognosis with each centimeter increase in tumour size, with no demonstrable difference in survival once the tumour size exceed 6 cm. Tumours with a size greater than 5 cm and less than 7 cm, aligned better with a T3 prognosis than a T2b. When the tumour exceeds 7 cm, the prognosis is similar to other T4 descriptors (Table 1) (4).
Full table
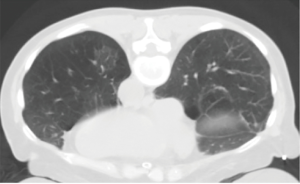
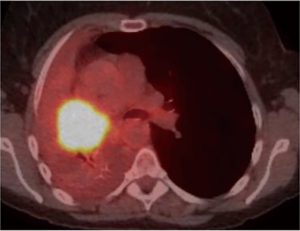
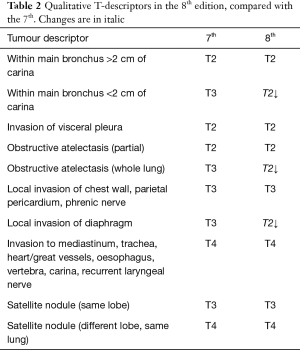
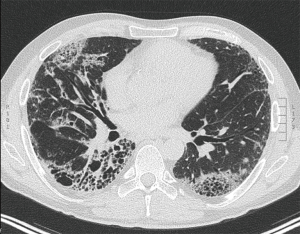
As for the other tumour descriptors, involvement of the main bronchus is more aligned with the prognosis of a T2 stage, regardless of its distance from the carina. This contrasts with the previous editions, where proximity to carina within 2 cm would be staged as T3 (8). Similarly, provided there is no mediastinal invasion, obstructive atelectasis is now staged as T2, regardless of whether it is the lobe or whole lung that is involved. In the 7th edition, whole lung involvement was staged as a T3. This change is more reflective of the prognostic value of the endobronchial growth of tumour (8) (Figure 1). On the other hand, invasion of the diaphragm is now T4 instead of T3, as the prognosis is poorer when assessed against other T3 descriptors (8) (Table 2, Figure 2). Mediastinal pleural invasion is difficult to define clinically, as it is not associated with clinical symptoms. At pathological staging, it is rare to find mediastinal pleural invasion without invasion of the mediastinal tissue, the latter being a T4 descriptor. As such, mediastinal pleural invasion has been removed from the T subset (8). The changes to the T descriptors for the 8th edition are highlighted in Tables 1,2.
Full table
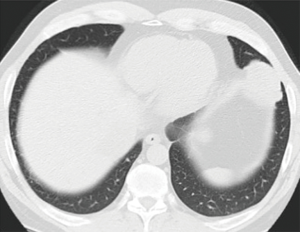
Subsolid nodules
Since the 7th edition of TNM was released, new entities of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) have been introduced (9). In contrast to previous editions, the 8th edition is the first to provide detailed recommendations on the radiologic staging of subsolid nodules, as well as the pathologic staging of what are broadly considered their histologic correlates. Of worthy note, the term ‘bronchioloalveolar carcinoma’ is no longer in the lung tumour lexicon. The radiologic staging depends on the presence and size of the solid component, and less so on the size of the overall nodule. Also, in contrast to the solid nodules which are measured to the nearest cm, these are measured to the nearest mm (e.g., 0.6 cm).
Ground glass nodules that measure less than 5 mm in maximal dimension are typically considered to be atypical adenomatous hyperplasia in which the malignant potential is still debatable and hence are not assigned a T descriptor at present. Pure ground glass nodules from 0.6–3 cm can be radiologically staged as cTis (clinical adenocarcinoma in-situ). A pure ground glass nodule >3 cm are considered lepidic predominant adenocarcinoma (LPA) and hence is staged as a cT1a (LPA) (9) (Figure 3). Part-solid nodules which are overall less than or equal than 3.0 cm, with a solid component of 5mm or less, are considered cT1mi (minimally invasive) whilst part-solid nodules which are larger than 3 cm with a solid component of less than 5 mm are considered cT1a. If the solid components of the part solid nodules are larger than 5 mm, the T staging is determined by the diameter of the solid component such that diameters of up to 1 cm, 2 cm and 3 cm are cT1a, cT1b and cT1c respectively (9).
There is general agreement between ground-glass opacity on CT with lepidic pattern on pathology, although this is not absolute, and the clinical staging is subject to revision following pathologic assessment of specimen (10). Likewise, the solid component of a subsolid nodule usually corresponds to the invasive component of the adenocarcinoma although benign, fibrous scar or areas of atelectasis may contribute to the dimension of the solid component (9).
Multiple tumour nodules
In addition, for multifocal pulmonary adenocarcinoma with ground glass nodules, staging can be undertaken by applying the highest T descriptor, followed by the total number of nodules in parenthesis, followed by the N and M descriptors. For instance, if there are 3 lesions in total; 2 pure ground glass nodules <3 cm and the other, a subsolid nodule with a solid component of 7 mm, the T-stage of T1a [3] applies, with the ‘[3]’ indicating the total number of lesions (9) (Figure 4). If there are multiple subsolid nodules, ‘m’ may also be used instead, to denote ‘multiple’. When multiple tumour nodules are viewed as intrapulmonary metastases; for instance, if they share similar histopathologic features on biopsy, share similar radiologic features and intervening adenopathy is present, classification is based on the location of the nodules in relation to the primary tumour; T3 in same lobe, T4 in different ipsilateral lobe, and M1a if in the contralateral lung (11-13). This includes patients with invasive mucinous adenocarcinomas who tend to have a diffuse, pneumonic-type appearance that may present with multiple foci of consolidation (11) (Figure 5).
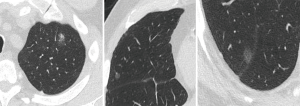
Troubleshooting
The IASLC Staging and Prognostic Factors Committee attempt to solve issues where data is limited, by means of literature review and consensus. Some of the recommendations drawn to facilitate homogenous classification in commonly-encountered clinical scenarios, are as outlined below:
Recurrent laryngeal nerve, superior vena cava (SVC), trachea and oesophageal involvement by tumour is considered T4. However, if these structures are involved by the way of ipsilateral nodal disease, they are staged N2 (8). Pancoast tumour arises from the superior pulmonary sulcus and is considered at least T3 given the invariable chest wall invasion. It can be assigned a higher T stage in the presence of invasion of the vertebral body, spinal canal, subclavian vessel encasement, or involvement above C8 brachial plexus (8). Adjacent lobe invasion and involvement of hilar fat is assigned a T2a, unless assigned a higher T category (8).
Nodal staging (N)
The nodal staging assesses tumour burden in the regional hilar and mediastinal nodes (7). The nodal stages have been able to consistently separate the patients into different prognostic group and remain unchanged from the earlier edition (Table 3). The nodal stations are as outlined in the IASLC nodal map developed in the 7th TNM edition (14). The explorative analysis of pathologically-classified cases suggested that prognosis can be more accurately defined if combined with the number of stations in N1 and N2 location. In short, the more nodal stations that are involved, the worse the prognosis. As such, a more precise subclassification of N1 into N1a (single station, ipsilateral hilum) and N1b (multiple stations); and N2 into N2a1 (single N2 station without N1 involvement), N2a2 (single N2 station with N1 involvement) and N2b (multiple N2 stations) were proposed for pathological staging.
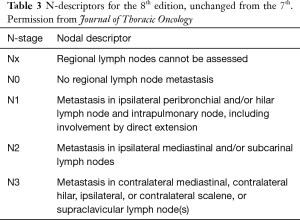
Full table
Detailed analysis of the pathologically classified cases shows that the 5-year survival rates in the population of M0 patients who underwent complete resection for the different N subcategories are: N1a, 59%; N1b, 50%; N2a1, 54%; N2a2, 43%; and N2b, 38%. A discrete mediastinal nodal disease without N1 disease has the same prognosis as multiple N1 station involvement (15).
The clinical quantification of nodal disease includes anatomic and metabolic images, transbronchial, endobronchial and transoesophageal needle aspiration, as well as mediastinoscopy or thoracoscopy. The clinical staging has not been able to reproduce the same data as the pathological nodal staging and hence, the clinical N staging has not been redefined (15). Whilst there is no change to the clinical staging of N disease, it is still recommended that the nodal station involvements are specified when possible, to allow refinement of post-operative prognosis and to facilitate future studies (15).
Metastasis staging (M)
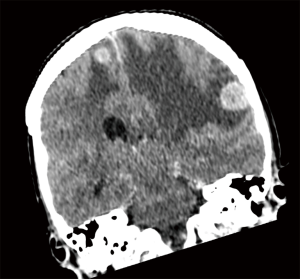
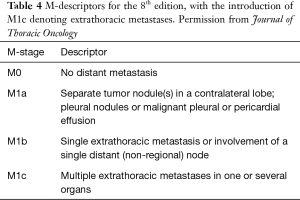
M staging is defined by the presence of metastasis beyond the regional lymph nodes. In the 8th edition, this data is extracted and analysed from the 1,059 patients that were prospectively registered through the electronic data capture (EDC) system (14). The analysis validated the 7th edition definition of intrathoracic metastatic disease, and this definition has thus been retained (14). However, when considering extrathoracic metastases, patients with single extra-thoracic metastasis have better prognosis than those with several metastases, with a mean survival of 11.4 months instead of 6.3 months, regardless of whether it is single or multi-organ involvement. Hence, M1b is further categorized to M1b and M1c to help better define oligometastasis (14) (Figure 6). Furthermore, it can also help define the group of patients for whom aggressive local therapy, in addition to systemic treatment, is more suitable (Table 4).
Full table
M1a tumours have similar prognosis as M1b, although they are kept separate as are likely to require different treatment and diagnostic approaches (4). By consensus from the IASLC Staging and Prognostic Factors Committee, for purposes of clarification and uniformity in reporting, pleural disease is considered M1a. However, if a metastatic lesion is outside the pleura, involving chest wall or contralateral diaphragm, it is considered extra-thoracic metastasis (4).
Stage grouping
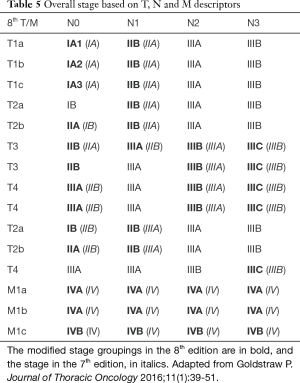
There is also further modification in the stage groupings, to accommodate for the new T and M classifications, and to separate the groups with significantly different survival outcome (16). This would affect the treatment options in certain patients. Stage Ia is now subclassified into Ia1, Ia2 and Ia3 to reflect the new T1a, T1b and T1c subcategorization. Similarly, stage IV is now subdivided into IVa and IVb; the IVb to reflect multiple extrathoracic metastases. In addition, stage IIIC is added to reflect the more locally advanced disease categories with T3 or T4 tumour, associated with N3 disease. This stage reflects on the worse prognostic outcome than seen in cases involving tumours that remain in stage IIIB. The downstaging of an endobronchial lesion from T3 to T2, is also reflected in the downward shift of the stages. The changes are as outlined in the Table 5. There are significant differences in survival between the stages, except for stage IIIc and IVa, which remains separated as they represent different forms of disease extent; between locoregional and metastatic disease (16).
Full table
Limitations of the new classification
One of the major limitation is that as a lot of the data collected was still not necessarily designed for TNM classification, the data lacks the necessary details or clarification for various, specific descriptors (6,17). One such example is lymphangitis carcinomatosis, which has not been incorporated into the clinical staging system as there is a lack of data to support it. The data is only available for registered patients prospectively through the electronic data capture, and not for those registered retrospectively. Of these patients, only 69 of NSCLC in the dataset had lymphangitis, and firm conclusions could not be drawn from the survival analysis obtained from the limited number (18,19). The standard uptake value (SUV) of individual lung tumours has not been incorporated for the same reasons. Moreover, the present database has no information on immunohistochemistry and molecular genetics such as epidermal growth factor receptor (EGFR) mutation status. Therefore, its prognostic impact could not be commented on, in the present dataset. In the era of precision medicine, sensitizing mutation such as EGFR can impact on treatment response and outcome. These may be incorporated in future staging systems (20). On this same note, more data will also be needed to guide the significance and staging of non-draining nodes such as the internal thoracic nodes.
As alluded to earlier, between the 7th and the 8th edition, there has been geographical variation in the data collected. There is still a deficiency in global distribution of the data. Certain regions such as North America and Africa and highly populate countries such as India and China are underrepresented whist others, notably Japan, are overrepresented. There is a higher prevalence of early lung cancer in the Asian demographics, and a later stage in the European countries, probably owing to the advent of lung cancer screening program in the former (17). However, multivariate analysis performed was adjusted for geographical region to compensate for this variable.
As with previous editions, most of the patients were treated with surgery, with little data on patients that were treated by radiotherapy and chemotherapy alone (n=157, 4.7%, decreased from previous 12%). As such, the generalisability of the new staging across non-surgical treatment modalities, and in turn, the prognostic impact of the TNM descriptors, may differ depending on the treatment applied (21). More studies are already on the way for external validation of the 8th edition TNM.
The importance of accurate measurement of the tumour size cannot be stressed enough for the 8th edition (9). Unfortunately, there is moderate inter and intraobserver variability when reporting the size of lung nodules, and this is worse in the context of subsolid nodules, or smaller lesions (22). Other technical or patient-related parameters such as slice thickness, reconstruction kernel and patients’ level of inspiration at time of acquisition, has also been reported to contribute to the variability in size of nodules (9). This area has been extensively researched, and multiple recommendations such as using lung windows, thin 1mm slices, and measuring the longest dimension on multiplanar and 3-D imaging is recommended. The utilization of volumetric measurement is also recommended in order to narrow the degree of variability and may be considered in future iterations of classification (9). More work would be needed for the standardization of image acquisition, measurement and reporting, to ensure a more consistent and reproducible report. It is important to note for now, that the volumetric measurement for subsequent follow up studies need to be measured using the same software, for better reproducibility. There is also variability and uncertainty with regards to how subsolid nodules should be measured. It is important to note that whilst the Fleischner criteria used for incidental lung nodules, recommends subsolid nodules to be measured in bi-dimensional, orthogonal planes and the value averaged (23), the TNM guidelines dictate that only the greatest average dimension used, where the long and short-axis measurements can be in axial or non-orthogonal planes. Finally, there are differences in opinion, about how best to measure ground glass nodules containing more than one region of solid component. Pathologic staging dictates that the solid or invasive components are summed. For reproducible clinical staging at this time, the recommended method is to measure the lesion with the largest dimension (9).
Impact of the new staging system on patient management
The data collected for the new TNM staging system is much richer and detailed compared to its predecessors. It allowed refinement of analysis of different descriptors that were not possible previously and is expected to help stratify patients and prognosis more accurately (4). The new edition should be used in trials of novel therapies, with more detailed descriptors to be used, to ‘study-proof’ the data for future reference. The new TNM staging also highlights the importance of multidisciplinary meetings as the standard of practice, in the comprehensive staging of patients with lung cancers. It better clarifies differences in the role between clinical, radiologic and pathologic staging, particularly when it comes to subsolid nodules and multiple pulmonary tumours. It must be noted that refining the staging does not dictate treatment, as the relation is not necessarily direct. Changes to established treatment should be based on clinical judgement and prospective trials (4).
Between the 7th and 8th edition, there is an overall observed improved survival when adjusted by stage.
This may be from improved staging accuracy with increasing availability of PET and other sampling techniques as outlined above, and improvement in various treatment options from adjuvant therapy following resection, to stereotactic therapy (16). This is particularly true with the PET which is increasingly being utilized in most developed countries, in the anatomical staging of lung cancer. Various studies have shown that as many as up to 62% of patient have had TNM stage changed following PET imaging, in which up to 52% resulted in change in management (7).
Conclusions
The 8th edition defines tumour size and establishes a new category for metastasis categories. It also confirms the prognostic relevance and further revalidates nodal staging. This helps create a more robust staging system to ensure consistent collection of data, which will ultimately help influence prognostic stratification, treatment decisions, as well as future data collection for research. For the radiologists involved in clinical staging of the lung cancer, there are several parameters that will require additional consideration. The most important are accuracy and consistency in reporting the tumour size, particularly with the subsolid nodules, as every centimeter now counts towards a higher T. Whilst the N staging remains unchanged, documenting the nodal stations involved may help future studies on prognostication. For M staging, it is now important to quantify the number of extrathoracic metastases. The new staging system is expected to positively impact the management of patients with lung cancer, and it is important for the clinicians as well as radiologists to be abreast of the new staging criteria to prepare for its international implementation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Cancer Research UK. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Zero, accessed Dec 2017.
- Rami-Porta R, Bolejack V, Giroux DJ, Chansky K, Crowley J, Asamura H, Goldstraw P. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members and Participating Institutions. The IASCL Lung Cancer Staging Project: The new database to inform the eight edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer-major changes in the American Joint Committee on Cancer eight edition staging manual. CA Cancer J Clin 2017;67:138-55. [Crossref] [PubMed]
- Goldstraw P, Crowley J. IASLC International Staging Project. The IASLC International Staging Project on Lung Cancer. J Thorac Oncol 2006;1:281-6. [Crossref]
- Rami-Porta R, Goldstraw P. Strength and weakness of the new TNM classification for lung cancer. Eur Respir J 2010;36:237-9. [Crossref] [PubMed]
- Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol 2012;4:128-34. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, Rice T, Suzuki K, Thomas CF Jr, Travis WD, Wu YL, Staging IASLC. Prognostic Factors Committee. Advisory Boards and Participating Institutions. IASLC Staging and Prognostic Factors Committee, Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revisions of the T descriptors in the forthcoming eight edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990-1003. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, Goo JM, MacMahon H, Naidich D, Nicholson AG, Powell CA, Prokop M, Rami-Porta R, Rusch V, van Schil P, Yatabe Y; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee and Advisory Board Members.The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Lim HJ, Ahn S, Lee KS, Han J, Shim YM, Woo S, Kim JH, Yie M, Lee HY, Yi CA. Persistent pure ground-glass nodules >10mm at CT; histopathologic comparisons. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Detterbeck FC, Franklin WA, Nicholson AG, Girard N, Arenberg DA, Travis WD, Mazzone PJ, Marom EM, Donington JS, Tanoue LT, Rusch VW, Asamura H, Rami-Porta R; IASLC Staging and Prognostic Factors Committee; Advisory Boards; Multiple Pulmonary Sites Workgroup. The IASLC Lung Cancer Staging Project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:651-65.
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017;9:269-79.
- Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, Goldstraw P, Rami-Porta R. International Association for Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming (8th) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball D, Rami-Porta R; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R, Staging IASLC. Prognostic Factors Committee. Advisory Boards, and Participating Institutions. The IASLC Lung Cancer Staging Project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eight) edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Singh N, Baldi M, Behera D. Inclusion of lymphangitis as a descriptor in the new TNM staging of lung cancer: filling up the blank spaces. J Thorac Oncol 2015;10. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V. Reply to “Inclusion of lymphangitis as a descriptor in the new TNM staging of lung cancer: Filling up the blank spaces”. J Thorac Oncol 2015;10:e119-20. [Crossref] [PubMed]
- El Masri J, Ren S, Zhang J. Getting familiar with the forthcoming eight edition of TNM classification of lung cancer from the T to N and M descriptors. Ann Transl Med 2016;4:67. [PubMed]
- Choi HS, Jeong BK, Jeong H, Lee YH, Ha IB, Song JH, Kang KM. Application of the new 8th TNM staging system for non-small cell lung cancer: treated with curative concurrent chemoradiotherapy. Radiat Oncol 2017;12:122. [Crossref] [PubMed]
- van Riel SJ, Sánchez CI, Bankier AA, Naidich DP, Verschakelen J, Scholten ET, de Jong PA, Jacobs C, van Rikxoort E, Peters-Bax L, Snoeren M, Prokop M, van Ginneken B, Schaefer-Prokop C. Observer variability for classification of pulmonary nodules on low-dose CT images and its effect on nodule management. Radiology 2015;277:863-71. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, Van Schil PE, Bankier AA. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]