Correlations between the abdominal fat-related parameters and severity of coronary artery disease assessed by computed tomography
Introduction
With the development of preventive medicine, the morbidity of coronary artery disease (CAD) is under control to a certain extent; however, the mortality of CAD remains a major problem worldwide (1-4). Besides, obesity is considered a challenging public problem (5), which has been proven to be closely associated with CAD (6-10). Numerous anthropometric adiposity measurements have been commonly used to evaluate obesity, including waist circumference (WC), body mass index (BMI), waist to height ratio (WHtR), and waist to hip ratio (WHR). However, these exhibited a weaker association between these values and risk factors for CAD unless people present with abundant visceral fat (11-13). Current researchers have reported the importance of fat distribution in cardiac metabolism (14).
Ectopic fat deposit refers to excessive adipose tissue located in an unusual position which involves visceral and subcutaneous region, muscle, and the abdominal organs (14,15). Visceral adipose tissue (VAT), being an endocrine organ, can secrete adipokines including cytokines and chemokines (14). It can also lead to insulin resistance and vascular inflammation, playing a role in obesity-mediated CAD (11,16-19). VAT is associated with cardiovascular disease and can be used as a cardiometabolic risk marker (20), while subcutaneous adipose tissue (SAT) shows the beneficial metabolic impact on the contrary (11,13,16,20-22). The VAT/SAT ratio is a unique parameter, which is relevant to vascular inflammation or cardiac events (13,16,20). Other abdominal parameters such as attenuation of other abdominal parenchymatous organs and abdominal aortic calcification (AAC) represent the abdominal fat distribution (23-34). Therefore, the combination of the above-mentioned risk factors may provide a better understanding of the association between the abdominal fat-related parameters and severity of CAD.
The purpose of this study is to comprehensively and quantitatively analyze whether the abdominal fat-related parameters are associated with severity of CAD using abdominal non-enhanced computed tomography (NECT).
Methods
Study design and study population
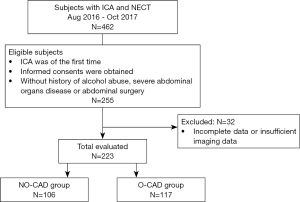
A study flowchart is shown in Figure 1. In-patients who went through both abdominal non-enhanced CT (NECT) and invasive coronary angiography (ICA) from August 2016 to October 2017 were retrospectively analyzed, and the examination of ICA should be of the first time. Informed consents were obtained from all participants. Traditional cardiovascular risk factors such as current smoking, diabetes mellitus, hypertension, high-density lipoprotein-cholesterol (HDL-C), and other baseline characteristics were collected. Patients with incomplete information and those with a history of alcohol abuse, severe abdominal organs disease, or abdominal surgery were excluded.
Evaluating the severity of coronary artery stenosis with ICA
ICA was examined with a standard technique, in which the diameter stenosis (DS) of each major epicardial coronary artery was assessed using quantitative coronary angiography (QCA) by two of 10-year-experienced cardiologists who were blinded to patients’ characteristics. In case of disagreement, a consensus was reached after consultation. All of the main coronary arteries, including left anterior descending artery (LAD), left circumflex (LX), and right coronary artery (RCA), were evaluated. Obstructive CAD (O-CAD) was defined as DS ≥50% in at least one of the main coronary arteries.
Measurements of the abdominal fat-related parameters
The abdominal fat-related parameters were acquired with abdominal NECT scan (Sensation 64, Siemens, Forchheim, Germany; Discovery CT750 HD and GE revolution, GE Healthcare, Milwaukee, WI, USA) using 120 kVp, 65 mAs, and 5-mm slide thickness. The images were captured in the supine position.
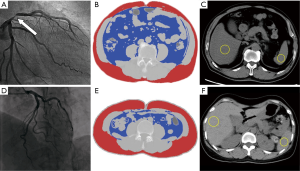
The abdominal perimeter was measured at the level of the umbilicus. The abdominal adipose tissue area VAT, total adipose tissue (TAT), and SAT were quantified utilizing a semi-automated tool of ImageJ 1.52e (National Institute of Health, Bethesda, MD, USA) at the level of the umbilicus (29). VAT was calculated by manually tracing the intra-abdominal wall muscular layer with the attenuation threshold ranging from −190 to −30 HU. TAT was also measured at the level of the umbilicus with the same density threshold, and SAT was quantified by deducting VAT from TAT (21,30,35). With these two parameters, the VAT/SAT ratio was then calculated (Figure 2). Nonalcoholic fatty liver disease (NAFLD) was defined as the ratio of liver-to-spleen attenuation<1 (L/S ratio). Regions of interest (ROIs) with an area of over 2 cm2 were placed at different axial levels of the liver and the spleen in the same axial image correspondingly, large vessels or biliary structures in the liver should be avoided (30). L/S ratio was obtained using mean CT value of the liver divided by that of the spleen. The ratio of pancreas-to-spleen (P/S ratio) was defined as the mean attenuation of the head, body, and tail of the pancreas divided by that of the spleen in the same axial slice. CT attenuation of the psoas major muscle was recorded as the average value of bilateral measurements at the level of the umbilicus. According to the abdominal NECT aortic calcification marking system (32,33), a reformed method was defined. An AAC score was acquired from L1 to L4 intervertebral levels. Once a patchy calcification appeared in a vertebral segment, the system would mark down one score, whereas a single and very tiny fleck of calcification would not be counted; hence, the total scores ranged from zero to four. All the image analysis and assessment were operated by a 3-year-experienced radiologist blinded to patient information. The intra-observer reliability was determined by repeated measurements on 30 patients, and its consistency was evaluated using the intra-class correlation coefficient (ICC).
Statistical analysis
All statistical analyses were performed using SPSS 19.0. Categorical variables were shown as the frequency, and the differences between two groups were tested using Pearson’s χ2 test. Continuous variables were demonstrated as mean ± standard deviation or median with interquartile range as appropriate, and the comparison between two groups was conducted using the independent sample t-test. For further evaluating the special contributions of the abdominal fat-related parameters with CAD, the multivariate logistic regression analysis was used to screen out independent risk factors of O-CAD after considering traditional cardiovascular risk factors and other CT variables in the study. ICC was used to assess the intra-observer reliability. The receiver-operating characteristic (ROC) curve was used for evaluating the predicting ability of these independent risk factors alone and the combination of them all for discriminating O-CAD, and the MedCalc statistical analysis was used to differentiate their predicting abilities. The statistical significance for all analyses were P<0.05.
Results
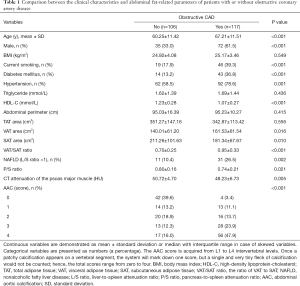
A total of 223 consecutive subjects (48% males; from 34 to 90 years old; mean age 63.90±11.96 years) who were suspect of having CAD and received ICA and abdominal NECT examinations subsequently were retrospectively analyzed. They were divided into two groups, NO-CAD group (n=106; 48%) and O-CAD group (n=117; 52%). The clinical characteristics and abdominal fat-related parameters of patients were listed in Table 1. A good intra-observer agreement was achieved with an ICC value of 0.91 by repeated measurements on 30 patients.
Full table
Traditional cardiovascular risk factors, including age, sex, current smoking, diabetes mellitus, hypertension, and HDL-C, were analyzed. Compared with NO-CAD subjects, patients with O-CAD were older (67.21±11.51 vs. 60.25±11.42 years, P<0.001), having a higher prevalence of male (P<0.001), current smoking (P<0.001), diabetes mellitus (P<0.001), and hypertension (P=0.001), and own lower HDL-C (1.07±0.27 vs. 1.23±0.28, P<0.001). Although BMI, triglyceride (TG), and abdominal perimeter reflected no significant differences between the two groups, the corresponding values in patients with O-CAD were higher than those with NO-CAD (25.17±3.46 vs. 24.92±4.08; 1.69±1.44 vs. 1.62±1.39; 95.23±10.27 vs. 95.03±16.39).
The abdominal fat-related parameters, including VAT area, SAT area, VAT/SAT ratio, L/S, P/S, and CT attenuation of the psoas major muscle as well as AAC score were calculated. VAT and VAT/SAT ratio in O-CAD patients were significantly greater than that in the NO-CAD group (161.53±61.54 vs. 140.01±61.20, P=0.016; 0.95±0.33 vs. 0.70±0.25, P<0.001). The AAC score in O-CAD patients were higher than that in NO-CAD patients (P<0.001). Moreover, patients in the O-CAD group demonstrated lower SAT, P/S, and CT attenuation of the psoas major muscle compared with the NO-CAD group (181.34±67.67 vs. 211.26±101.63, P=0.010; 0.74±0.21 vs. 0.80±0.16, P=0.021; 48.23±6.73 vs. 50.72±4.70, P=0.005).
The multivariable logistic regression analysis in Table 2 shows that the VAT/SAT ratio [OR, 10.492; 95% confidence interval (CI), 3.130–35.170], NAFLD (OR, 2.807; 95% CI, 1.135–6.941), and AAC score (OR, 1.964; 95% CI, 1.550–2.487) are independent predictors of O-CAD after adjusting traditional cardiovascular risk factors. Diabetes mellitus (OR, 2.572; 95% CI, 1.164–5.682), a traditional cardiovascular risk factor, is still a predictor of O-CAD after the adjustment in the multivariable model.

Full table
The ROC curve analysis illustrated distinctive predicting abilities for O-CAD with independent risk factors alone and the combination of them all (Figure 3). The predicting abilities for independent risk factors of NAFLD, diabetes mellitus, VAT/SAT ratio, and AAC score were 0.58 (95% CI, 0.51–0.66), 0.62 (95% CI, 0.54–0.69), 0.73 (95% CI, 0.67–0.80), and 0.77 (95% CI, 0.71–0.83), respectively, beyond doubt, AAC score is a better predictor of those three. The predicting ability of the combination of the above four factors is 0.85 (95% CI, 0.79–0.90). The statistical analysis demonstrated a higher ability of the combination of all risk factors than that of each single of them independently (P<0.05).

Discussion
In this study, we comprehensively assessed the correlations of the abdominal fat-related parameters and clinical characteristics between NO-CAD and O-CAD patients. Our study indicated that the factors including age, sex, current smoking, diabetes mellitus, hypertension, HDL-C, VAT, SAT, VAT/SAT ratio, NAFLD, P/S ratio, CT attenuation of the psoas major muscle, and AAC score are correlated with the severity of CAD. The multivariable logistic regression analysis shows the VAT/SAT ratio, NAFLD, and AAC score are independent risk factors of O-CAD after adjusting traditional cardiovascular risk factors. Diabetes mellitus, a traditional cardiovascular risk factor, remains as an independent predictor in the model. The most attractive result is that a doubling in the VAT/SAT ratio is accompanied by 10.49-fold hazard of the O-CAD; thus, it is a CT-derived parameter which may contribute to the better recognition of patients with increased risk of O-CAD and to whom more attention should be paid.
At present, a few studies have separately evaluated the correlations between VAT and cardiometabolic risks, between SAT and cardiometabolic risks, between VAT/SAT ratio and vascular inflammation or cardiac events, and between NAFLD and risks of cardiovascular disease (11,13,16,18,20-29). Several studies also suggest that AAC score is correlated with CAD (30-34). However, they only focused on one of these factors at a single time which is not comprehensive enough and it might potentially cause bias (16,20,27-29,31,32). In fact, in our study, a comprehensive quantitative assessment is performed between the abdominal fat-related parameters (including TAT, VAT, SAT, VAT/SAT ratio, L/S, P/S, CT attenuation of the psoas major muscle, and AAC score) and severity of CAD. Thus, the findings of this study add valuable information to the current literature.
In clinical practice, BMI and abdominal perimeter can be used to evaluate risk stratification of CAD. In the current investigation, although the values of them in patients with O-CAD are higher than that in NO-CAD subjects, there is no significant difference in these values between the two groups, suggesting a weak correlation with CAD (12,13). Therefore, our results do not validate previous anthropometric adiposity measurements including BMI and abdominal perimeter.
Adipose tissue has been considered an effective modulator. Normally, it is an organ where fat accumulates, in which the adipocytes can maintain lipid homeostasis by increasing their number and dimensions (15,18). Previous studies showed that VAT is metabolically active which can lead to systemic inflammation and insulin resistance by secreting adipokines (14,16-18). Prospective data are in favor of VAT being an independent risk factor of CAD. As Zamboni et al. (15) demonstrated in their study, the effect of SAT was protective (16,18,19), and SAT, being the first “buffer system” which can offset circulating dietary fat when overeat, can also deliver a satiety message to the hypothalamus according to secreting leptin. However, the roles of VAT and SAT concerning cardiovascular health are still controversial. In this case, a better and more accurate marker for the impact of fat distribution is needed. In our study, VAT of the O-CAD patients is higher while SAT is lower than that of the NO-CAD patients, and the statistical difference is significant, indicating that VAT is harmful while SAT is protective. Therefore, the VAT/SAT ratio serves a relatively more accurate and powerful index of distribution of abdominal fat than absolute quantification of VAT or SAT does independently. Decreased CT attenuations of liver, pancreas, and psoas major muscle represent fat deposits in the abdominal organs. NAFLD has been closely related to significant coronary stenosis detected by ICA; it is also relevant to coronary arteries and abdominal arteries calcification. The prevalence of NAFLD shows obesity-associated ectopic fat deposit which is further correlated with CAD. Previous studies and a recent meta-analysis reveal that AAC score is correlated with CAD and is also a predictor of O-CAD (30-34); thus, AAC score has been used as a marker of atherosclerosis and risk assessment of CAD. It is generally accepted that the plaque in abdominal aorta is the early-stage atherosclerosis. As the disease progresses, the plaque will calcify eventually (32). The result shows the correlations between AAC and O-CAD. In summary, our study comprehensively quantifies the correlations between severity of CAD and each of abdominal fat-related parameters, clinical characteristics, and traditional cardiovascular risk factors, respectively, to more accurately reflect their contributions.
Abdominal NECT is noninvasive and can provide accurate quantification of the abdominal CT parameters. Moreover, it is inexpensive and convenient to acquire the images with no contrast injection required. Most importantly, the radiation dose is lower than CT angiography and ICA. Therefore, it is a practice approach to predict the severity of CAD using abdominal NECT parameters.
One identified limitation of our study is the nature of being a single-center and small-sample one; therefore, a larger sample and multi-center research are needed. In addition, it owns a cross-sectional design. What is more, the time bias between ICA and abdominal NECT and whether the drug therapy was administrated are hardly controlled, and these confounding factors might influence the outcomes. Thus, prospective studies are necessary to support the roles of these indicators in clinical practice. The DS of ≥50% was used to determine significant stenosis in this study which may not be accurate as the current approach to define significant coronary stenosis is >70% DS, while the DS between 50% and 69% is regarded as intermediate stenosis. However, this study focuses on the correlation between the fat-related parameters and severity of CAD rather than coronary lumen stenosis. Thus, results are valid which allow us to draw robust conclusions.
Conclusions
The abdominal fat distribution related CT parameters of VAT/SAT ratio and NAFLD, and AAC score are correlated with severity of CAD, and they become independent risk factors for O-CAD after adjusting traditional cardiovascular risk factors. Diabetes mellitus, as a traditional cardiovascular risk factor, is still included as an independent predictor. These indicators are conducive to identifying patients with increased risk of O-CAD. Besides, the combination of all the independent risk factors increases the ability for predicting O-CAD than each factor alone.
Acknowledgements
Funding: This research was supported by the National Nature Science Foundation of China (NSFC, no. 81525014), the Jiangsu Provincial Special Program of Medical Science (BL2013029) and the Key Research and Development Program of Jiangsu Province (BE2016782).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by IEC for Clinical Research of Zhongda Hospital, Affiliated to Southeast University (No. 2017ZDKYSB086). Written informed consent was obtained from all patients.
References
- Sun Z. Quantitative cardiovascular imaging. Quant Imaging Med Surg 2014;4:297-9. [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146-e603. [Crossref] [PubMed]
- D'Errico L, Salituri F, Ciardetti M, Favilla R, Mazzarisi A, Coppini G, Bartolozzi C, Marraccini P. Quantitative analysis of epicardial fat volume: effects of scanning protocol and reproducibility of measurements in non-contrast cardiac CT vs. coronary CT angiography. Quant Imaging Med Surg 2017;7:326-35. [Crossref] [PubMed]
- O’Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol 2008;61:299-310. [Crossref] [PubMed]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491-7. [Crossref] [PubMed]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925-32. [Crossref] [PubMed]
- Fifth Joint Task Force of the European Society of Cardiology. European Association of Echocardiography. European Association of Percutaneous Cardiovascular Interventions; European Heart Rhythm Association; Heart Failure Association; European Association for Cardiovascular Prevention & Rehabilitation; European Atherosclerosis Society; International Society of Behavioural Medicine; European Stroke Organisation; European Society of Hypertension; European Association for the Study of Diabetes; European Society of General Practice/Family Medicine; International Diabetes Federation Europe; European Heart Network. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Prev Cardiol 2012;19:585-667.
- Kim SH, Despres JP, Koh KK. Obesity and cardiovascular disease: friend or foe? Eur Heart J 2016;37:3560-8. [Crossref] [PubMed]
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105-20. [Crossref] [PubMed]
- Corrigan FE 3rd, Kelli HM, Dhindsa DS, Heinl RE, Al Mheid I, Hammadah M, Hayek SS, Sher S, Eapen DJ, Martin GS, Quyyumi AA. Changes in truncal obesity and fat distribution predict arterial health. J Clin Lipidol 2017;11:1354-1360.e3. [Crossref] [PubMed]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39-48. [Crossref] [PubMed]
- Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KA, Smith SC Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM. International Day for the evaluation of abdominal obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007;116:1942-51. [Crossref] [PubMed]
- McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756-60. [Crossref] [PubMed]
- Wu FZ, Wu CC, Kuo PL, Wu MT. Differential impacts of cardiac and abdominal ectopic fat deposits on cardiometabolic risk stratification. BMC Cardiovasc Disord 2016;16:20. [Crossref] [PubMed]
- Zamboni M, Rossi AP, Fantin F, Budui SL, Zoico E, Zamboni GA, Mazzali G. Predictors of ectopic fat in humans. Curr Obes Rep 2014;3:404-13. [Crossref] [PubMed]
- Hong HC, Hwang SY, Park S, Ryu JY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Kim S, Choi KM. Implications of pericardial, visceral and subcutaneous adipose tissue on vascular inflammation measured using 18FDG-PET/CT. PLoS One 2015;10. [Crossref] [PubMed]
- Wensveen FM, Jelenčić V, Valentić S, Šestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Štimac D, Wunderlich FT, Brüning JC, Mandelboim O, Polić B. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 2015;16:376-85. [Crossref] [PubMed]
- Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol 2011;31:2715-22. [Crossref] [PubMed]
- Marques MD, Santos RD, Parga JR, Rocha-Filho JA, Quaglia LA, Miname MH, Avila LF. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis 2010;209:481-6. [Crossref] [PubMed]
- Ladeiras-Lopes R, Sampaio F, Bettencourt N, Fontes-Carvalho R, Ferreira N, Leite-Moreira A, Gama V. The ratio between visceral and subcutaneous abdominal fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Rev Esp Cardiol 2017;70:331-7. [Crossref] [PubMed]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997;46:1579-85. [Crossref] [PubMed]
- Goel K, Misra A, Vikram NK, Poddar P, Gupta N. Subcutaneous abdominal adipose tissue is associated with the metabolic syndrome in Asian Indians independent of intra-abdominal and total body fat. Heart 2010;96:579-83. [Crossref] [PubMed]
- Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular disease and myocardial abnormalities in nonalcoholic fatty liver disease. Dig Dis Sci 2016;61:1246-67. [Crossref] [PubMed]
- Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O'Donnell CJ, Speliotes EK. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol 2015;63:470-6. [Crossref] [PubMed]
- McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: The Diabetes Heart Study. Am J Gastroenterol 2008;103:3029-35. [Crossref] [PubMed]
- Lim S, Oh TJ, Koh KK. Mechanistic link between nonalcoholic fatty liver disease and cardiometabolic disorders. Int J Cardiol 2015;201:408-14. [Crossref] [PubMed]
- Puchner SB, Lu MT, Mayrhofer T, Liu T, Pursnani A, Ghoshhajra BB, Truong QA, Wiviott SD, Fleg JL, Hoffmann U, Ferencik M. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology 2015;274:693-701. [Crossref] [PubMed]
- Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016;65:1136-50. [Crossref] [PubMed]
- Osawa K, Miyoshi T, Yamauchi K, Koyama Y, Nakamura K, Sato S, Kanazawa S, Ito H. Nonalcoholic hepatic steatosis is a strong predictor of high-risk coronary-artery plaques as determined by multidetector CT. PLoS One 2015;10. [Crossref] [PubMed]
- Fox CS, Hwang SJ, Massaro JM, Lieb K, Vasan RS, O'Donnell CJ, Hoffmann U. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study). Am J Cardiol 2009;104:543-7. [Crossref] [PubMed]
- An C, Lee HJ, Lee HS, Ahn SS, Choi BW, Kim MJ, Chung YE. CT-based abdominal aortic calcification score as a surrogate marker for predicting the presence of asymptomatic coronary artery disease. Eur Radiol 2014;24:2491-8. [Crossref] [PubMed]
- Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart 2012;98:988-94. [Crossref] [PubMed]
- Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV. Abdominal Aortic Calcification Detected on Lateral Spine Images From a Bone Densitometer Predicts Incident Myocardial Infarction or Stroke in Older Women. J Bone Miner Res 2008;23:409-16. [Crossref] [PubMed]
- Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, Ouyang P, Criqui MH. Abdominal aortic calcium and multi-site atherosclerosis: the multiethnic study of atherosclerosis. Atherosclerosis 2011;214:436-41. [Crossref] [PubMed]
- Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211:283-6. [Crossref] [PubMed]


