The role of radioprotective spacers in clinical practice: a review
Introduction
Radiotherapy remains one of the curative treatment modalities for localized or loco-regionally advanced malignancies, second only to surgery. The radiation dose delivered to the tumor has a direct impact on treatment efficacy and patient survival. In clinical practice, however, the prescribed dose is often a trade-off between tumor control probability (TCP) and normal tissue complication probability (NTCP). NTCP is the major dose-limiting factor in radiotherapy.
To keep NTCP as low as possible, fractionated radiation schemes are used to protect normal tissues to some degree. The underlying therapeutic mechanism is the discrepancy in radiosensitivity and damage repair between the normal and cancerous tissues. Radiobiologically, researchers have also explored radioprotective pharmaceuticals to reduce NTCP. Agents such as amifostine (1,2) and acetylsalicylic acid (3) have been reported to protect normal tissues from free radical damage, but their clinical efficacy has been equivocal, and their efficacy regarding dose escalation in radiotherapy is limited.
Though recent advances in RT delivery, including image-guided RT (IGRT) and intensity modulated radiation therapy (IMRT) have led to reduced toxicity rates, it has proven a challenge to protect normal tissue from radiation injuries because there are occasions when some critical organs are located too close to the tumor. This makes it virtually impossible to deliver a curative radiation dose with sufficient space for dose fall-off and organ-sparing. It holds true even in particle therapy or iridium-based brachytherapy (BT) where a characteristically sharp fall-off of the beams can be achieved. To allow for maximal tumor radiation dose while limiting exposure to the immediate adjacent organs at risk (OARs), the method available is to manually displace these OARs so that they are located at some distance from the tumor. This can be accomplished by introducing a spacer between them.
The aim of this literature review is to analyze and summarize the findings regarding the utilization of radioprotective spacers in radiotherapy with emphasis on the following: the history of development; the materials used; the clinical applications and future directions.
Statement of search strategies used and sources of information
A systematic search of the PubMed, Web of science and the Cochrane Library databases was carried out. Using individual keywords or combinations of the keywords ‘spacer’, ‘radioprotection’, ‘radiotherapy’, ‘radiation injuries’, ‘organ at risk’, ‘normal tissue complication probability’, ‘toxicities’, ‘hydrogel’, and ‘balloon’. Specific therapeutic names were also searched, such as ‘prostate cancer’, ‘cervical cancer’, ‘head and neck cancer’, ‘digestive system neoplasm’, ‘intensity-modulated radiotherapy’, ‘stereotactic body radiation therapy’, and ‘brachytherapy’.
History of spacers
The clinical utilization of spacers in radiotherapy was first reported in 1984; when a removable pelvic spacer was used in abdominopelvic neoplasia to prevent radiation damage to the intestinal loops (4). In the same year, Niwa et al. used a spacer in a tongue cancer patient during interstitial BT to prevent osteoradionecrosis of the mandible (5). Since then, there has been an increasing number of studies on utilization, mainly involving tongue cancer and abdominopelvic cancer radiotherapy. In the 1990s, Sezeur et al. (6-8) conducted several clinical studies involving the application of a silicone balloon and proved its feasibility and safety in patients with abdominopelvic malignancy. Fujita et al. (9-11) reported the role of spacers in reducing osteoradionecrosis after interstitial BT in tongue cancer patients. Spacer utilization was limited at that time.
For cervical carcinoma, an important concern was the potential late toxicities regarding the bladder and rectum during high-dose-rate (HDR) BT. An intra-vaginal balloon catheter (12) was developed in 2004. Before this balloon application, Gauge packing was an important choice for cervical cancer to alleviate rectal toxicities. The ability of this device to decrease radiation complications at low cost made the procedure cost effective and customizable in HDR BT for cervical cancers.
More recently, research concerning spacer utilization has been focused on prostate cancer radiotherapy. After the first study involving hyaluronic acid (HA) as a spacer in prostate radiotherapy by Prada et al. in 2007 (13), there has been a rapidly increasing number of spacer studies including different spacer materials on this single disease entity (14-19).
Materials of spacers
Spacer materials used in radiotherapy depend on the anatomical site of the tumor, and are being constantly developed with the advancement in chemical engineering (Table 1). The use of the following spacer materials have been reported in various anatomical sites.
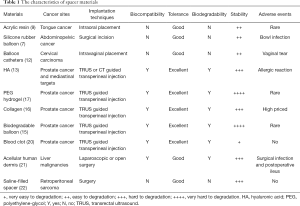
Full table
Silicone and acrylic resin
The spacer material first used in tongue cancer radiotherapy consisted of radiation-permeable silicone; its protective effect was demonstrated 30 years ago (5). Later on, acrylic resin spacers were used in interstitial BT for tongue cancer (9,23). In 2003, Obinata et al. (24) reported a clinical study involving spacers composed of hard plaster and irreversible hydrocolloid material; they were comfortable to wear and easily adjusted by physicians.
Blood patch
Morancy et al. (20) evaluated the blood patch as a spacer in three prostate cancer patients receiving LDR BT, The blood patch technique involved injecting 20 mL of the patient’s blood between the rectum and the prostate and produced an average separation distance of 3.86 mm. Although the rectal volume receiving ≥100 Gy (V100) was reduced from 3.44 to 0 mL, the durability of the blood patch spacer is poor. With increasing use of hypofractionated radiotherapy or stereotactic body radiotherapy (SBRT) where treatment duration can be shortened considerably, the blood patch might be re-emerged as an option because of its good accessibility and biocompatibility.
Balloon
Initially designed for abdominal cancers, a temporary prosthesis consisting of an expandable silicone balloon was implanted and filled with saline before radiotherapy. This balloon could displace the intestines from the radiation field and it was deflated and removed through a 3-cm incision at the completion of treatment because of its non-biodegradable characteristic (7,8). In addition to the silicone balloon, biodegradable balloons developed by Prospace are prepared from poly (L-lactide-co-epsilon-caprolactone) and they remain inflated during radiotherapy; they can be degraded and absorbed after treatment (15,25). The implantation of a biodegradable balloon requires a needle, a dilator and an introducer sheath (25). The balloon system allows for positional correction if needed. In addition, the balloon does not have a limited handling time in the initial implantation step and its visibility using computed tomography (CT) and magnetic resonance imaging (MRI) is satisfactory. Subsequently, the balloon-packing system consisting of two balloons was developed (Bracco Diagnostics Inc., Princeton, New Jersey, USA) (26), and various filling materials including air, water, 100% contrast medium, 100% normal saline and a 1:1 solution of normal saline and contrast medium were used to test its characteristics. The majority study on this balloon were in uterine cervix carcinoma. Each balloon was typically filled with 30 to 50 cc of solution depending on the vaginal size. Saini et al. (26) tested different filling materials in the balloon to reduce the radiation dose to the bladder and rectum during BT. The balloon filled with iodine solution was well-performed in comparison to saline solution or air. In addition, the dose reduction effect was dependent on the contrast agent concentration of the solutions.
Human collagen
Human collagen has been used as a tissue expander in various body sites. Noyes et al. (16) described human collagen as a spacer that could be used to increase the distance between the prostate and the anterior rectal wall. As a commercially available human collagen product, marketed as Cymetra, the effectiveness of the collagen was documented as well as the changes in radiation dose to the rectum. Before the start of IMRT (≤75.6 Gy), 11 patients received 20 mL of human collagen which was injected into the perirectal space using a transperineal approach; the mean reduction in dose to the anterior rectal wall was 50% and no rectal symptoms were reported. However, due to the tendency of the collagen to be lumped, it was difficult to obtain the desired consistency when using larger volumes. On the market, human collagen was commonly packaged; it needs to be reconstituted with sterile saline. Beyond that, the availability of human collagen is fluctuant and dependent on the economic rules of supply and demand. The above reasons may limit the application of collagen as a spacer in cancer radiotherapy.
HA
HA is a naturally occurring glycosaminoglycan-based polymer found in connective tissue and extracellular matrix. Prada et al. (13) reported that HA injections could be used as the interval materials to prevent rectal damage in prostate cancer radiotherapy. Characteristically, HA can remain stable in the prostate-rectum space for 12 months without changing the shape and position. Kishi et al. (27) also developed a new high-molecular-weight hyaluronic gel as a spacer to safely displace the esophagus away during interstitial radiotherapy of a mediastinal target close to the esophagus. This gel was prepared as a mixture of 10 mL of hyaluronan and 0.8 mL of iodine contrast media, and the volume was enough for an effective esophageal shifting from a target point. However, artificially cross-linked HA may cause antibody production and a long-lasting allergic reaction (13,28). In addition, HA does not appear to be radiation stable with degradation within weeks after radiation exposure (29). Due to its viscous nature, HA may not distribute evenly and thus be of limited clinical application.
Polyethylene-glycol (PEG) hydrogel
PEG hydrogel is most frequently used as radioprotective spacers in prostate cancer (17,18,30,31). SpaceOAR™ (Augmenix Inc., Waltham, MA) is the only Food and Drug Administration (FDA) approved hydrogel rectal commercially available in Europe and US. Being water soluble, non-immunogenic and non-toxic, absorbable PEG hydrogels are injected as liquids and polymerize in situ in <10 s following the mixture of two precursor solutions. The PEG spacer will maintain its integrity for 3 months prior to degradation, and hydrolysis via renal metabolism will occur within 6 months. Pinkawa et al. (18,31-34) performed a series of Phase II clinical studies using PEG as the spacer material. They validated and characterized the spatial and dosimetric effects of PEG hydrogel, and evaluated safety and efficacy regarding the reduction of late toxicities after 12 months. Physically, the gel was stable during the course of radiotherapy and not detectable on MRI after 9–12 months because of complete absorption. More recently, this retrospective cohort study (34) with 114 prostate cancer patients received EBRT to evaluate QOL changes up to 5 years after RT with this hydrogel spacer application. This study clearly showed the dosimetric advantage and long-term clinical benefits in protecting the rectum from high dose levels throughout the treatment duration.
Acellular human dermis
A study was reported on seven liver cancer patients who underwent biologic mesh spacer (BMS) placement followed by high dose radiotherapy (21). This clinical trial was conducted at the University of Texas MD Anderson Cancer Center between 2012 and 2013 (21). All patients selected for BMS placement were labeled as having “unresectable liver tumors”. The BMS was derived from cadaveric human skin that was chemically treated to preserve the biologically active dermal matrix. It displaced the bowel, allowing for the delivery of higher doses of radiation to liver tumors. Following irradiation, none of the patients developed upper gastrointestinal (GI) bleeding or a peptic ulcer and there were no complication-related re-admissions. Despite BMS provided a survival benefit, the mesh used in this study is expensive and its image in CT or MRI was likely to radiologists for tumor surveillance.
Saline-filled spacer
In a retrospective cohort study (22), a saline-filled spacer was inserted in retroperitoneal sarcoma (RPS) patients before postoperative radiation therapy. This study proved that it was feasible to displace adjacent radiosensitive structures using an intraoperatively placed spacer. This placement minimized toxicity associated with postoperative radiation therapy. And evidence suggests that the potential survival advantage conferred by leaving spacers outweighs the risk of spacer placement (22).
Application of spacer in patients with cancer
Prostate cancer
Spacer has now become an important instrument in prostate cancer radiotherapy. Naturally, the anterior rectal wall is located close to the prostate and is difficult to be spared of the high-dose irradiation. If a spacer is injected or inserted between the prostate and anterior rectal wall to achieve a distance, then the rectum receives a lower dose, thus diminishing the probability of treatment-related side effects. Several studies have analyzed spacer application in prostate cancer with emphasis on RT delivery, materials and QOL of patients. In EBRT for prostate cancer, Pinkawa et al. (35) evaluated the spacer dimensions and prostate position variability during the full course of EBRT. They found that distances between the prostate and anterior rectal wall were well maintained at all levels, and the protection of the rectum was achieved throughout the treatment course. In a prospective multicenter phase II trial, Uhl et al. (30) evaluated the safety, and clinical effects of the PEG hydrogel prostate-rectum spacer at 3, 6 and 12 months following irradiation. According to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) criteria, the incidence of acute or late gastrointestinal (GI) and genitourinary (GU) toxicities was reduced in prostate cancer patients receiving IMRT. The feasibility and effectiveness of the hydrogel injection were proved. Another randomized controlled trial (36) involving a total of 222 patients was performed to evaluate spacer placement in patients receiving IMRT at doses of ≤79.2 Gy; the proportion of spacer applications rated as “easy” or “very easy” reached 98.7% accompanied with a 99% success rate. Furthermore, overall late rectal adverse event rates were significantly reduced and no late rectal toxicity greater than grade 1 was found in the spacer group. Recently, this prospective single-blind randomized phase III trial (37) reported the final results with a median follow-up period of 3 years. They demonstrated that the hydrogel spacer resulted in a significant rectal dose reduction and a substantial portion of patients benefited from the use of the spacer. Encouragingly, they also provided the first level 1 evidence to prove that the benefit seen with the hydrogel spacer in decreasing the bowel toxicity. QOL at 15 months was maintained or improved at the 3-year median follow-up period.
In a Hypofractionated Radiotherapy regimen (20 fractions of 3.1 Gy) with injection of HA Chapet et al. (38) reported that 19 prostate cancer patients experienced grade 2 toxicities with urinary obstruction and/or frequency. No grade 3 or 4 toxicities occurred. A prospective analysis (39) involving 76 patients with clinical stages of T1–T3a prostate cancer was reported on the dosimetric benefits, spacer safety, and late GI toxicities following injection of a hydrogel spacer. The results showed that the volume of rectum receiving ≥70 Gy is less than 12%, and no patients experienced acute or late grade 2+ GI toxicities. Most recently, a study assessed the use of implanted hydrogel rectal spacers for stereotactic ablative radiotherapy (SABR)-volumetric modulated arc therapy (SABR-VMAT) patients with prostate cancer, and found the rectal volume receiving 36 Gy reduced by ≥42% for all patients and the median NTCP for Grade 2 + rectal bleeding significantly decreased from 4.9% to 0.8% with the use of a rectal spacer (P=0.031) (40). The above studies come to one conclusion that injection of hydrogel spacer is beneficial for prostate cancer patients undergoing radiotherapy.
In BT for prostate cancer, Wilder et al. (28) prospectively analyzed whether cross-linked hyaluronan gel could reduce the rectal radiation dose and the occurrence of acute rectal toxicity. There was no acute diarrhea in ten patients who received a spacer. Prada et al. (41) published the first report on HDR BT using a spacer. The implantation procedure was well tolerated in all patients and no intraoperative or perioperative complications occurred. Taggar et al. (42) assessed the impact of placing a rectal hydrogel spacer on dosimetry and acute toxicity immediately following a LDR BT using Pd-103 seed-implant. Seventy four patients had placement of a hydrogel spacer and 136 patients did not. At the time of the first follow up, no patients experienced grade 2 GI toxicities. Prostate and urethral dosimetries were not affected by the placement of a spacer. Wu et al. (43) have assessed the utility and dosimetric effect of SpaceOAR (Augmenix, Inc., Waltham, MA) in eighteen patients scheduled for HDR BT in the treatment of prostate cancer. As compared to the 36 patients treated with HDR BT without spacers, patients who received SpacerOARs had a significantly lower dose to the rectum.
Rucinski et al. (44) investigated daily rectal dose reduction using a gel spacer in carbon ion therapy for prostate cancers, and concluded that the risk of rectal toxicity was substantially reduced. With technical innovations, particle therapy has also become increasingly accessible for the treatment of a variety of tumors with distinct dosimetric and radiobiological advantages. Further investigation for the safety and other characteristics is needed to explore spacer applications in this modality of therapy.
Cervical cancer
Both external beam radiotherapy and BT are the current standard modalities of treatment for locally advanced cervical carcinoma. Gastrointestinal toxicities, which can greatly affect QOL of patients, are urgent concerns associated with the treatment. The method of spacer placement for dose sparing to the rectum and bladder in cervical cancer radiotherapy has gained recognition several decades ago. Eng et al. (12) reported on the potential dose reduction to bladder and rectum using intravaginal Foley balloon catheters during HDR BT for cervical cancer. A study reported (45) that three patients with locally recurrent gynecologic malignancies requiring re-irradiation underwent hydrogel spacer placement (Duraseal; Covidien, Mansfield, MA). The addition of the gel spacer alone significantly reduced rectal, sigmoid, and small-bowel doses and sufficiently protected the surrounding tissues to permit reirradiation with curative doses. Damato et al. (46) performed a study to evaluate the TraceIT hydrogel (TH; Augmenix, Waltham, MA) spacer, a novel iodinated polyethylene glycol between the cervix, rectum, and bladder during cervical cancer radiation in five female cadavers compared with the current standard of gauze packing. And, they found using of hydrogel resulted in a 22% decrease in rectum D2cc dose (P=0.02), a 10% decrease in bladder D2cc (P=0.27) (46).
Head and neck cancer
The use of the first radiolucent silicone spacer was reported in 1984 during interstitial BT for cancer of the tongue (5). A distance of 8 mm was generated between the lower gingiva and the tongue lesion, which allowed the average radiation dose (time dose fraction) to the lower gingiva to be as low as 45% of the minimal tumor dose. Later in 1998, a retrospective analysis including 103 patients with T1 or T2 tongue carcinoma was conducted by Miura et al. (23) to evaluate the efficacy of spacers in the prevention of mandibular complications in LDR BT alone or with EBRT. The absolute incidence of osteoradionecrosis was 2.1% (1/48) with and 40.0% (22/55) without a spacer. More recently, Rao et al. (47) demonstrated the feasibility of displacing the contralateral submandibular gland using a hydrogel injection technique. This procedure enabled substantial reduction in both volume and dose irradiated to the gland. The mean dose to the targeted submandibular gland decreased from 40.4 Gy pre-injection to 25.4 Gy post-injection for an average reduction of mean dose by 16.4 Gy (47).
Thoracic and digestive system neoplasm
Kishi et al. (27) reported two cases of recurrent mediastinal tumor with gel injected to safely displace the esophagus from the high dose area during HDR BT. The accumulated esophageal D1cc (minimum dose to the most irradiated volume of p cc. minimum dose to the most irradiated volume of 1 cc) was remarkably reduced in spacer patients as compared to those without spacers. There was no evidence of recurrences or late complications observed after the procedure. Thus, this technique might be used in conjunction with boost irradiation or reirradiation to the upper mediastinal targets to spare the esophagus from radiation injuries. For unresectable hepatocellular carcinoma, Komatsu et al. (48) devised a two-step treatment approach with surgical spacer placement and subsequent proton therapy, which enabled the delivery of a curative dose to the target without treatment-related toxicities. In addition, the spacer has also been assessed in tumors arising in the head of the pancreas (19), for the purpose of improving the outcomes in patients with unresectable pancreatic cancers.
Other cancer
There were also a few studies of spacer utilization in cancers of some remaining sites of the body. A removable prosthesis was introduced in a clinical study involving 22 patients with retroperitoneal or pelvic cancers who received postoperative EBRT (4). No modifications in biological parameters were observed during pelvic radiotherapy at 30, 45 and 65 Gy, and the small intestine tolerated the treatment well. In a study involving re-irradiation of recurrent cancer using HDR BT (49), the organ-sparing effect and safety profile were evaluated in 30 patients with lesions in the head and neck, breast, skeleton, axilla, pelvis and abdominal wall. The authors found that the NTCP decreased from 60.8%±12.6% to 16.1%±19.8% (P<0.01). Takahashi et al. (50) presented a case with a huge sacral chordoma treated using a similar method and the outcome was also satisfactory. This two-step treatment regimen might provide a cure for those tumors that are otherwise incurable using radiotherapy because of their extreme proximity to sensitive organs. Similarly as described above, a saline-filled spacer (22) was reported in the treatment of a retroperitoneal sarcoma to maximize the oncological benefit.
Procedure-related complications
According to the published literature, most spacer implantation is well tolerated. However, a limited number of studies have reported complications related to the procedure or the device itself. In general, these complications are associated with implantation technique and the anatomical sites of tumors. For abdominopelvic malignancies, Sezeur et al. (6-8) reported that one patient suffered a small bowel wound during the positioning of the device, leading to an infection and subsequent removal of the device. Another patient had vaginal leakage related to postsurgical ascites caused by a major hepatectomy. In the pelvis, two cases involving retroperitoneal placement of the prosthesis had to be deflated between EBRT sessions because of flank pain, whereas most patients only experienced transient heaviness after saline injection into the balloon. In a randomized study of patients with cervical carcinoma treated with HDR BT (51), one patient was reported to have a minor vaginal tear as a result of over-inflation of the bladder-rectum spacer balloon.
In multi-institutional phase II trials (30,52) for prostate cancer, one patient experienced a device-related event of grade one proctitis and three patients had procedure-related complications of focal rectal mucosal necrosis from inadvertent injection of hydrogel into the rectal wall; the bladder was pierced leading to hydrogel leakage into the bladder and urinary retention. Chapet et al. (38) reported three patients who experienced lower abdomen pain, hematuria and asthenia after the injection of HA, and one patient developed a hematoma between the rectum and the bladder. Wilder et al. (28) emphasized that the main risk associated with injection of cross-linked hyaluronan gel was infection, the occurrence of infectious prostatitis caused by puncture is related to whether or not prophylactic antibiotic regimens were administered, and the use of prophylactic antibiotic therapy is estimated to reduce the risk of infection to <5%. To avoid the occurrence of a hematoma, Chapet et al. (38) highlighted the importance of screening for the potential risk of clotting disorders. For rectal spacer implantation, acute rectal toxicity including rectal discomfort and bleeding is usual. One patient who received SpaceOAR developed a perineal abscess 1 month after treatment (43). To date, no mortality has been reported involving the spacer placement procedure in clinical practice.
Discussion
Evolution of spacer development
It has been over 30 years since the first report of radioprotective spacer utilization in clinical practice. Initially, spacers were used mainly for interstitial BT of tongue cancers, and the number of publications in this anatomical site reached its peak in 2000 and then dropped sharply. This possibly reflects the fact that with increasing use of IMRT, BT for tongue cancer is no longer prevalent and concerns about osteoradionecrosis have been largely mitigated. In stark contrast, spacer use in prostate cancer radiotherapy has been rapidly expanding over the past 10 years. This can be explained by the growing number of prostate cancer patients being treated with radiotherapy and concerns over rectal toxicity because of extreme prostate-rectum proximity. One more reason for the widespread use of spacers in prostate cancer is the relative ease of implantation and good safety profiles. The spacer, whether it consists of balloons or hydrogels, can be easily introduced into the potential space between the Denonvilliers fascia and the anterior wall of the rectum under ultrasound guidance. In addition, the stability of the spacer can be maintained throughout the course of treatment.
Paradigm-changing role of radioprotective spacer in oncology
Patients and medical workers can choose the most suitable treatment through multiple disciplinary team (MDT), and avoid unnecessary treatment, so as to reduce patients’ mental suffering and cost of patient care. Apart from prostate and cervical cancers, little progress has been made in spacer use in other pelvic cancers and in anatomical sites such as the retroperitoneum. This can be explained by the fact that in these parts of the body, it is difficult to implant a spacer and maintain its optimal shape and position. For locally advanced and obviously unresectable tumors, integrative work from all experts (after MDT discussion) is helpful to find out optimal strategies for patients. If necessary, a surgical intervention can be performed prior to radiotherapy to obtain pathological samples, to mobilize the OARs away from the lesion and then position a spacer in between. This intervention can be done via an open incision, endoscopically, and laparoscopically, preferably making a minimally invasive procedure. The hypothesized advantage of the new combination lies in the fact that a suboptimal operation cannot eradicate the tumor but rather increase the possibility of cancerous dissemination. And curative radiation dose can be safely delivered with a surgically placed spacer between the OAR and the tumor in such complex anatomical sites as the thorax, abdomen and pelvis. More robust studies are needed to demonstrate the value of this so-called pre-radiotherapy operation (PRO), in which radiotherapy plays a definitive role in cancer therapy.
SBRT and the spacer
SBRT, or SABR, is being increasingly applied in the treatment of a variety of cancers. Because of the extremely large fraction sizes used, SBRT kills cancer cells more efficiently and irreversibly. But it makes the protection of the adjacent normal tissues more important because of the large fraction size. The use of a spacer can be a solution. King et al. (40) reported the first study to assess the dosimetric impact, NTCPs and early toxicity in SBRT of the prostate cancer using a hydrogel spacer. The spacer insertion in all six patients was successful under local anesthesia. Substantial reduction in Grade 2 + rectal bleeding and Grade 1 GI toxicity was observed with the use of a rectal spacer following treatment. In addition to the prostate, SBRT has also become a promising treatment modality for locally advanced pancreatic cancer. However, this practice has been limited to a few specialized centers due to the risk of duodenal toxicity. In a cadaveric study (19), the feasibility and theoretical dosimetric advantages of hydrogel separation of the pancreatic head from the duodenum were proven. Spacer application not only decreased the total but also the fractionated dose received by the OARs during SBRT. Instead of the month-long treatment needed in conventionally fractionated radiotherapy. SBRT requires considerably less time for a treatment course. Conceivably, a spacer for SBRT can be designed for shorter durability with better patient compliance, which potentiates its wider future application.
Conclusions
Spacer application reduces NTCP and increases the probability of cure in cancer radiotherapy. More robust studies are warranted to further explore the role of spacers, which can make radiotherapy more safe and effective.
Acknowledgements
Funding: This study was supported by grant from Natural Science Foundation of Zhejiang Province (grant number: LY16H160013), by grant from the health and family planning commission of Zhejiang Province (grant number: 2018KY063) and by grant from Chinese Medicine Research Program of Zhejiang Province (grant number: 2018ZZ014).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ormsby RJ, Lawrence MD, Blyth BJ, Bexis K, Bezak E, Murley JS, Grdina DJ, Sykes PJ. Protection from radiation-induced apoptosis by the radioprotector amifostine (WR-2721) is radiation dose dependent. Cell Biol Toxicol 2014;30:55-66. [Crossref] [PubMed]
- Felice PA, Nelson NS, Page EE, Deshpande SS, Donneys A, Rodriguez J, Buchman SR. Amifostine Reduces Radiation-Induced Complications in a Murine Model of Expander-Based Breast Reconstruction. Plast Reconstr Surg 2014;134:551e-60e. [Crossref] [PubMed]
- Demirel C, Kilciksiz SC, Gurgul S, Erdal N, Yigit S, Tamer L, Ayaz L. Inhibition of Radiation-Induced Oxidative Damage in the Lung Tissue: May Acetylsalicylic Acid Have a Positive Role? Inflammation 2016;39:158-65. [Crossref] [PubMed]
- Dürig M, Steenblock U, Heberer M, Harder F. Prevention of radiation injuries to the small intestine. Surg Gynecol Obstet 1984;159:162-3. [PubMed]
- Niwa K, Morita K, Kanazawa H, Yokoi M. Usefulness of a radiolucent spacer in radiation therapy for cancer of the tongue. Gan No Rinsho 1984;30:1861-5. [PubMed]
- Sezeur A, Martella L, Abbou C, Gallot D, Schlienger M, Vibert JF, Touboul E, Martel P, Malafosse M. Small intestine protection from radiation by means of a removable adapted prosthesis. Am J Surg 1999;178:22-5; discussion 25-6. [Crossref] [PubMed]
- Sezeur A, Abbou C, Chopin D, Rey P, Leandri J. Protection of the small intestine against irradiation by means of a removable prosthesis. ASAIO Trans 1990;36:M681-3. [PubMed]
- Sezeur A, Abbou C, Rey P, Leandri J, Maurel J, Baudot P, Faggianelli F, Malafosse M. New surgical procedure for the protection of the small intestine before postoperative pelvic irradiation. Ann Chir 1990;44:352-5. [PubMed]
- Tamamoto M, Fujita M, Yamamoto T, Hamada T. Techniques for making spacers in interstitial brachytherapy for tongue cancer. Int J Prosthodont 1996;9:95-8. [PubMed]
- Fujita M, Hirokawa Y, Tamamoto M, Kashiwado K, Akagi Y, Kashimoto K, Wada T. Dose-reducing effect of Lipowitz metal-embedded spacers in interstitial brachytherapy for carcinoma of the mobile tongue. Oral Surg Oral Med Oral Pathol 1994;77:589-93. [Crossref] [PubMed]
- Fujita M, Tamamoto M, Hirokawa Y, Kashiwado K, Akagi Y, Kashimoto K, Wada T. Experimental and clinical studies on dose reduction effects of spacers in interstitial brachytherapy for carcinoma of the mobile tongue. Oral Surg Oral Med Oral Pathol 1993;76:797-803. [Crossref] [PubMed]
- Eng TY, Fuller CD, Cavanaugh SX, Blough MM, Sadeghi A, Herman T. Significant rectal and bladder dose reduction via utilization of Foley balloon catheters in high-dose-rate tandem and ovoid intracavitary brachytherapy of the uterine cervix. Int J Radiat Oncol Biol Phys 2004;59:174-8. [Crossref] [PubMed]
- Prada PJ, Fernández J, Martinez AA, de la Rúa A, Gonzalez JM, Fernandez JM, Juan G. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys 2007;69:95-102. [Crossref] [PubMed]
- Whalley D, Hruby G, Alfieri F, Kneebone A, Eade T. SpaceOAR Hydrogel in Dose-escalated Prostate Cancer Radiotherapy: Rectal Dosimetry and Late Toxicity. Clin Oncol (R Coll Radiol) 2016;28:e148-54. [Crossref] [PubMed]
- Melchert C, Gez E, Bohlen G, Scarzello G, Koziol I, Anscher M, Cytron S, Paz A, Torre T, Bassignani M, Dal Moro F, Jocham D, Yosef RB, Corn BW, Kovács G. Interstitial biodegradable balloon for reduced rectal dose during prostate radiotherapy: results of a virtual planning investigation based on the pre- and post-implant imaging data of an international multicenter study. Radiother Oncol 2013;106:210-4. [Crossref] [PubMed]
- Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1918-22. [Crossref] [PubMed]
- Weber DC, Zilli T, Vallee JP, Rouzaud M, Miralbell R, Cozzi L. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J Radiat Oncol Biol Phys 2012;84:e311-8. [Crossref] [PubMed]
- Pinkawa M, Corral NE, Caffaro M, Piroth MD, Holy R, Djukic V, Otto G, Schoth F, Eble MJ. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol 2011;100:436-41. [Crossref] [PubMed]
- Rao AD, Feng Z, Shin EJ, He J, Waters KM, Coquia S, DeJong R, Rosati LM, Su L, Li D, Jackson J, Clark S, Schultz J, Hutchings D, Kim SH, Hruban RH, DeWeese TL, Wong J, Narang A, Herman JM, Ding K. A Novel Absorbable Radiopaque Hydrogel Spacer to Separate the Head of the Pancreas and Duodenum in Radiation Therapy for Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2017;99:1111-20. [Crossref] [PubMed]
- Morancy TJ, Winkfield KM, Karasiewicz CA, Kaplan ID. Use of a blood-patch technique to reduce rectal dose during cesium-131 prostate brachytherapy. Int J Radiat Oncol 2008;72:S331-2. [Crossref]
- Ismael HN, Denbo J, Cox S, Crane CH, Das P, Krishnan S, Schroff RT, Javle M, Conrad C, Vauthey J, Aloia T. Biologic mesh spacer placement facilitates safe delivery of dose-intense radiation therapy: A novel treatment option for unresectable liver tumors. Eur J Surg Oncol 2016;42:1591-6. [Crossref] [PubMed]
- Reid J, Smith R, Borg M, Dobbins C, Gowda R, Chryssidis S, Borg M, Neuhaus S. Feasibility of spacers to facilitate postoperative radiotherapy for retroperitoneal sarcomas. J Med Imaging Radiat Oncol 2017;61:812-8. [Crossref] [PubMed]
- Miura M, Takeda M, Sasaki T, Inoue T, Nakayama T, Fukuda H, Hoshi A, Hoshina M, Shibuya H. Factors affecting mandibular complications in low dose rate brachytherapy for oral tongue carcinoma with special reference to spacer. Int J Radiat Oncol Biol Phys 1998;41:763-70. [Crossref] [PubMed]
- Obinata K, Ohmori K, Tuchiya K, Nishioka T, Shirato H, Nakamura M. Clinical study of a spacer to help prevent osteoradionecrosis resulting from brachytherapy for tongue cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:246-50. [Crossref] [PubMed]
- Kouloulias V, Kalogeropoulos T, Platoni K, Georgakopoulos J, Matsopoulos G, Chaldeopoulos D, Beli I, Pantelakos P, Asimakopoulos C, Kouvaris J, Kelekis N. Feasibility and radiation induced toxicity regarding the first application of transperineal implementation of biocompatible balloon for high dose radiotherapy in patients with prostate carcinoma. Radiat Oncol 2013;8:82. [Crossref] [PubMed]
- Saini AS, Zhang GG, Finkelstein SE, Biagioli MC. Dose reduction study in vaginal balloon packing filled with contrast for HDR brachytherapy treatment. Int J Radiat Oncol Biol Phys 2011;80:1263-7. [Crossref] [PubMed]
- Kishi K, Iida T, Ojima T, Sonomura T, Shirai S, Nakai M, Sato M, Yamaue H. Esophageal gel-shifting technique facilitating eradicative boost or reirradiation to upper mediastinal targets of recurrent nerve lymph node without damaging esophagus. J Radiat Res 2013;54:748-54. [Crossref] [PubMed]
- Wilder RB, Barme GA, Gilbert RF, Holevas RE, Kobashi LI, Reed RR, Solomon RS, Walter NL, Chittenden L, Mesa AV, Agustin J, Lizarde J, Macedo J, Ravera J, Tokita KM. Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:824-30. [Crossref] [PubMed]
- Daar E, King L, Nisbet A, Thorpe RB, Bradley DA. Viscosity changes in hyaluronic acid: irradiation and rheological studies. Appl Radiat Isot 2010;68:746-50. [Crossref] [PubMed]
- Uhl M, Herfarth K, Eble MJ, Pinkawa M, van Triest B, Kalisvaart R, Weber DC, Miralbell R, Song DY, DeWeese TL. Absorbable hydrogel spacer use in men undergoing prostate cancer radiotherapy: 12 month toxicity and proctoscopy results of a prospective multicenter phase II trial. Radiat Oncol 2014;9:96. [Crossref] [PubMed]
- Pinkawa M. Spacer application for prostate cancer radiation therapy. Future Oncol 2014;10:851-64. [Crossref] [PubMed]
- Pinkawa M, Klotz J, Djukic V, Schubert C, Escobar-Corral N, Caffaro M, Piroth MD, Holy R, Eble MJ. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology 2013;82:963-8. [Crossref] [PubMed]
- Pinkawa M, Piroth MD, Holy R, Escobar-Corral N, Caffaro M, Djukic V, Klotz J, Eble MJ. Quality of life after intensity-modulated radiotherapy for prostate cancer with a hydrogel spacer. Matched-pair analysis. Strahlenther Onkol 2012;188:917-25. [Crossref] [PubMed]
- Pinkawa M, Berneking V, Schlenter M, Krenkel B, Eble MJ. Quality of Life After Radiation Therapy for Prostate Cancer With a Hydrogel Spacer: 5-Year Results. Int J Radiat Oncol Biol Phys 2017;99:374-7. [Crossref] [PubMed]
- Pinkawa M, Piroth MD, Holy R, Escobar-Corral N, Caffaro M, Djukic V, Klotz J, Eble MJ. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol 2013;106:220-4. [Crossref] [PubMed]
- Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg S, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Hamstra DA, Bosch W, Gay H, Michalski J. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;92:971-7. [Crossref] [PubMed]
- Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg S, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Daignault-Newton S, Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys 2017;97:976-85. [Crossref] [PubMed]
- Chapet O, Decullier E, Bin S, Faix A, Ruffion A, Jalade P, Fenoglietto P, Udrescu C, Enachescu C, Azria D. Prostate hypofractionated radiation therapy with injection of hyaluronic acid: acute toxicities in a phase 2 study. Int J Radiat Oncol Biol Phys 2015;91:730-6. [Crossref] [PubMed]
- Chao M, Ho H, Chan Y, Tan A, Pham T, Bolton D, Troy A, Temelcos C, Sengupta S, McMillan K, Cham CW, Liu M, Ding W, Subramanian B, Wasiak J, Lim Joon D, Spencer S, Lawrenstchuk N. Prospective analysis of hydrogel spacer for prostate cancer patients undergoing radiotherapy. BJU Int 2018. Epub ahead of print. [Crossref] [PubMed]
- King RB, Osman SO, Fairmichael C, Irvine DM, Lyons CA, Ravi A, O'Sullivan JM, Hounsell AR, Mitchell DM, McGarry CK, Jain S. Efficacy of a rectal spacer with prostate SABR-first UK experience. Br J Radiol 2018;91. [Crossref] [PubMed]
- Prada PJ, Jimenez I, Gonzalez-Suarez H, Fernandez J, Cuervo-Arango C, Mendez L. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy 2012;11:105-10. [Crossref] [PubMed]
- Taggar AS, Charas T, Cohen GN, Boonyawan K, Kollmeier M, McBride S, Mathur N, Damato AL, Zelefsky MJ. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy 2018;17:251-8. [Crossref] [PubMed]
- Wu SY, Boreta L, Wu A, Cheung JP, Cunha JAM, Shinohara K, Chang AJ. Improved rectal dosimetry with the use of SpaceOAR during high-dose-rate brachytherapy. Brachytherapy 2018;17:259-64. [Crossref] [PubMed]
- Rucinski A, Brons S, Richter D, Habl G, Debus J, Bert C, Haberer T, Jäkel O. Ion therapy of prostate cancer: daily rectal dose reduction by application of spacer gel. Radiat Oncol 2015;10:56. [Crossref] [PubMed]
- Viswanathan AN, Damato AL, Nguyen PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol 2013;31:e446-7. [Crossref] [PubMed]
- Damato AL, Kassick M, Viswanathan AN. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound. Brachytherapy 2017;16:949-55. [Crossref] [PubMed]
- Rao AD, Coquia S, De Jong R, Gourin C, Page B, Latronico D, Dah S, Su L, Clarke S, Schultz J, Rosati LM, Fakhry C, Wong J, DeWeese TL, Quon H, Ding K, Kiess A. Effects of biodegradable hydrogel spacer injection on contralateral submandibular gland sparing in radiotherapy for head and neck cancers. Radiother Oncol 2018;126:96-9. [Crossref] [PubMed]
- Komatsu S, Hori Y, Fukumoto T, Murakami M, Hishikawa Y, Ku Y. Surgical spacer placement and proton radiotherapy for unresectable hepatocellular carcinoma. World J Gastroenterol 2010;16:1800-3. [Crossref] [PubMed]
- Kishi K, Sonomura T, Shirai S, Sato M, Tanaka K. Critical organ preservation in reirradiation brachytherapy by injectable spacer. Int J Radiat Oncol Biol Phys 2009;75:587-94. [Crossref] [PubMed]
- Takahashi M, Fukumoto T, Kusunoki N, Tsuchida S, Kido M, Takebe A, Awazu M, Kataoka Y, Matsumoto I, Miki T, Hori Y, Suzuki S, Kuroda D, Murakami M, Hishikawa Y, Ku Y. Particle beam radiotherapy with a surgical spacer placement for unresectable sacral chordoma. Gan To Kagaku Ryoho 2010;37:2804-6. [PubMed]
- Rai B, Patel FD, Chakraborty S, Kapoor R, Sharma SC, Kumaravelu S, Raghukumar P, Aprem AS. Bladder-Rectum Spacer Balloon versus Vaginal Gauze Packing in High Dose Rate Brachytherapy in Cervical Cancer: A Randomised Study (Part II). Clin Oncol (R Coll Radiol) 2015;27:713-9. [Crossref] [PubMed]
- Uhl M, van Triest B, Eble MJ, Weber DC, Herfarth K, De Weese TL. Low rectal toxicity after dose escalated IMRT treatment of prostate cancer using an absorbable hydrogel for increasing and maintaining space between the rectum and prostate: results of a multi-institutional phase II trial. Radiother Oncol 2013;106:215-9. [Crossref] [PubMed]


