Rigid body rotation of the left ventricle in hidradenitis suppurativa (a case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study)
Introduction
In healthy subject, the left ventricular (LV) apex rotates in counterclockwise direction, while the LV base shows a clockwise rotation resulting in a towel-wringing-like motion called LV twist (1). However, this is a very sensitive motion, which could be altered early by several pathological states. Under some special circumstances, the LV base and apex rotate in the same clockwise or counterclockwise direction resulting in the near absence of LV twist called as LV ‘rigid body rotation’ (RBR). Hidradenitis suppurativa (HS) is a dermatological disorder associated with long-standing chronic inflammation, its relationship with cardiovascular (CV) morbidity and mortality is documented (2).
The newly developed three-dimensional speckle-tracking echocardiography (3DSTE) has an ability to non-invasively characterize LV twist and to detect the presence of LV-RBR. With the present case, LV myocardial rotational mechanics was first examined in a HS patient without CV risk factors.
Case presentation
We report the case of a 32-year-old female patient with Hurley stage II HS who was involved in the Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases (MAGYAR-Path) Study. This study was organized in our tertiary center to determine clinical, diagnostic and prognostic importance of 3DSTE-derived parameters in different diseased states (“magyar” means “Hungarian” in Hungarian language). Informed consent was obtained from the patient, and the study protocol was approved by the institution’s human research committee.
This patient’s HS started 18 years ago at the age of 14 years. She has no other diseases. Family history is negative. Her body mass index is normal, rather in the lower range. Her symptoms—inflamed nodules, discharging abscesses, draining sinuses, later even scarring—started in the inguinal area, then years later, it progressed into the gluteal area. Fourteen years ago, she was started on ethinyl estradiol and cyproterone acetate, which she had been taking for 8 years. Her disease was stable while she was taking the drug, without apparent signs of activity. After this treatment, HS symptoms returned. Eighteen months ago, tetracycline treatment (500 mg bid) was administered for 3 months, then local clindamycin emulsion was used bid in the involved areas for 6 months. Now, she takes metformin 2×500 mg with low disease activity. Her routine laboratory tests are normal.
Complete two-dimensional (2D) Doppler and 3DSTE were carried out at the same time with the same commercially available Toshiba ArtidaTM echocardiography equipment (Toshiba Medical Systems, Tokyo, Japan). During 2D echocardiography, a 1–5 MHz matrix phased-array PST-30SBP transducer was used, while for 3DSTE it was changed for a 1–4 MHz matrix phased-array PST-25SX transducer for 3D data acquisition. Data analysis and chamber quantifications were performed in accordance with recent guidelines and practices (3,4). The Artida 3D Wall Motion Tracking software, version 2.7 was used for chamber quantifications. From the 3D echocardiographic pyramidal dataset, apical two- (2CH) and four-chamber (4CH) views and 3 short-axis views at different LV levels were automatically selected by the software at end-diastole (from the LV base to the LV apex). 2CH and 4CH views were used to manually mark the edges of the LV endocardium in the level of the mitral valve at each side and at the LV apex, then the software performed an automatic reconstruction of the 3D endocardial surface and tracked the endocardial surface in 3D space during the cardiac cycle (Figure 1). LV apical, midventricular and basal rotations and global unidirectional radial, longitudinal, circumferential, complex area, and 3D strains were calculated.
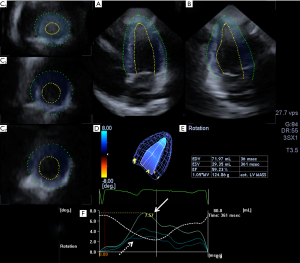
During 2D studies, normal chamber internal dimensions and functional parameters could be detected without any alterations (LV ejection fraction proved to be 59%). No significant valvular heart disease could be confirmed. 3DSTE-derived global LV radial, longitudinal, circumferential, area and 3D strains were 46.2%, −15.2%, −28.7%, −40.5% and 46.8%, respectively. Interestingly, LV apical and basal rotations were in the same counterclockwise direction confirming LV-RBR (Figure 1).
Discussion
Increased CV risk has been demonstrated in some dermatological diseases in recent decades including HS possibly due to chronic inflammation (5). HS was found to be associated with a significantly increased risk of adverse CV outcomes and all-cause mortality independent of measured confounders. The risk of CV-associated death was higher in patients with HS compared with the risk in those with severe psoriasis (2). Therefore, the early detection and treatment in HS patients may prevent late indirect complications, i.e., CV disease (6).
To the best of the authors’ knowledge, this is the first time to examine LV rotational mechanics in a single female patient with HS without CV risk factors in reproductive age. Although LV strain parameters, features of LV deformation proved to be normal, LV-RBR, the near absence of LV twist could be detected. LV twist has an essential part of ejection due to physiologic facts: if ejection was simply the result of the contraction of myocardial fibers, the EF would be 15–20%, whereas the actual LV-EF is 60–70% due to the involvement of twisting (1). However, LV-EF was normal in the presented case possibly due to compensating mechanisms.
Several special pathological states have been demonstrated where LV twist is missing: different cardiomyopathies, infiltrative disorders, congenital heart diseases, and other rare or unknown diseases (7). However, its occurrence in these disorders is not known. The most experiences are with noncompaction cardiomyopathy, where its presence is estimated to be between 55–100% in different studies (8,9). The pathological background of LV-RBR in HS is not known, but the role of chronic inflammation, oedema, and fibrosis could be assumed. However, perfusion abnormalities could not be excluded either. Further large volume studies are warranted to examine the real prevalence of LV-RBR in HS, and to investigate whether LV-RBR has a clinical, diagnostic or prognostic importance in this dermatological disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient, and the study protocol was approved by the institution’s human research committee.
References
- Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound 2011;19:1-6. [Crossref] [PubMed]
- Egeberg A, Gislason GH, Hansen PR. Risk of Major Adverse Cardiovascular Events and All-Cause Mortality in Patients With Hidradenitis Suppurativa. JAMA Dermatol 2016;152:429-34. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography -- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Godoy-Gijón E, Meseguer-Yebra C, Palacio-Aller L, Godoy-Rocati DV, Lahoz-Rallo C. New populations at increased cardiovascular risk: Cardiovascular disease in dermatological diseases. Clin Investig Arterioscler 2016;28:143-53. [PubMed]
- Shalom G. Hidradenitis suppurativa: epidemiology, clinical features, associated comorbidities and treatment. G Ital Dermatol Venereol 2017;152:46-57. [PubMed]
- Nemes A, Földeák D, Domsik P, Kalapos A, Sepp R, Borbényi Z, Forster T. Different patterns of left ventricular rotational mechanics in cardiac amyloidosis-results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2015;5:853-7. [PubMed]
- Peters F, Khandheria BK, Libhaber E, Maharaj N, Dos Santos C, Matioda H, Essop MR. Left ventricular twist in left ventricular noncompaction. Eur Heart J Cardiovasc Imaging 2014;15:48-55. [Crossref] [PubMed]
- Kalapos A, Domsik P, Forster T, Nemes A. Comparative evaluation of left ventricular function by two-dimensional echocardiography and three-dimensional speckle-tracking echocardiography in noncompaction cardiomyopathy. Results from the MAGYAR-Path Study. Orv Hetil 2013;154:1352-9. [Crossref] [PubMed]


