Clinical and imaging features of pituitary apoplexy and role of imaging in differentiation of clinical mimics
Introduction
Pituitary apoplexy (PA) is a rare serious and potentially fatal medical emergency with a vast spectrum of clinical manifestations. Most frequent symptom is sudden onset of severe retro-orbital headache (90–97%) (1-5). That is why PA is not often suspected initially because of similar presentation of more frequent diseases such as subarachnoid hemorrhage (SAH), cervical artery dissection or cerebral dural sinus thrombosis (6). The second most common symptom is the vision deficit (50% to 82%) (6-9). Other symptoms/signs include diminished consciousness and pan hypopituitarism (80%), nausea, vomiting, ophthalmoplegia and meningism (25–50%) (3,7,8). Pre-existing macro adenoma (65–90%), especially non-functioning and prolactinomas, are most susceptible to apoplexy which undergoes hemorrhage or infarct, but PA can occur with normal pituitary or micro adenoma (9-11).
PA occurs in1.6% to 2.8% of patients with adenoma and 0.2–0.6 events per 100 person-year in non-functioning pituitary adenomas (3-5,7,8). Usually, PA occurs in the middle age adults (typically 50–60 years) and is rare in children, with male preponderance (M:F =2:1) (7,8,12). Diagnosis of PA is frequently missed because, in addition to its relative rarity, clinical presentation can be acute or slowly progressive (subacute) which depend upon bleeding extent, edema extension, and necrotic evolution (13). Moreover, the existence of an adenoma is not often suspected at the time of PA episode because in more than 80% of the patients, PA is often the first presentation of an underlying pituitary tumor (especially nonfunctioning adenomas) (9,10). However, apoplexy can also occur in non-adenomatous lesions, such as craniopharyngioma (14), Rathke’s cleft cyst (RCC) (15), sellar tuberculoma (16), hypophysitis (17,18), sellar abscess (19), sellar metastasis (20), or even in normal pituitary gland during peri or post-partum period as a consequence of sever hypovolemic shock (Sheehan’s syndrome) (21).
The diagnosis of PA can be made only when hemorrhagic infarction of the pituitary gland leads to the above mentioned clinical syndrome. Small to large hemorrhages can be a common finding in up to 25% patients with macroadenomas without PA symptoms (1). The use of term PA should be avoided in asymptomatic patients, which is sometimes referred as subclinical PA (21). In one study, approximately one third of patients had non-hemorrhagic infarction of the adenoma, and these patients presented with less severe clinical symptoms and longer course before presenting for medical care than those who had hemorrhage or hemorrhagic infarction of the adenoma (22).
Pathophysiology
The incidence of intralesional bleeding in a pituitary adenoma is estimated five times higher than other intracranial neoplasms (1). The precise pathophysiology is still not completely understood. Under physiological conditions, the normal pituitary gland receives most of its blood supply through the capillary network of superior and inferior hypophyseal (portal) vessels in the infundibulum, and to a lesser degree, direct arterial blood supply (23). In addition, it is suggested that pituitary gland might have a critical perfusion pressure well below normal arterial pressure (24). It is possible that this peculiar vascularity and sudden alterations in perfusion pressure by various triggering factors (Table 1) (25-37), may predispose to sudden hemorrhage and/or infarction in micro adenomas as well as non-adenomatous lesions. Diabetes and arterial hypertension do not predispose patients to PA as reported in previous studies (16).
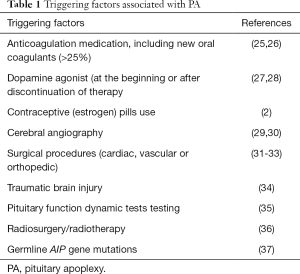
Full table
In pituitary adenomas, direct arterial source is generally more dominant than normal pituitary gland and thus may be directly influenced by an alteration in systemic arterial pressure (23). In addition, pituitary adenoma vessels display immature morphology of poor fenestration, ruptured and fragmented basal membranes (38). Various triggering factors as mentioned in Table 1 can be identified only in about 40% of PA in adenomas, and in the absence of triggering factors, intrinsic vasculopathy possibly contributes to the susceptibility for spontaneous hemorrhage (1). Moreover, dynamic imaging and histopathology have shown that macroadenomas, as well as microadenomas, are less vascularized than the pituitary gland (39,40). Thus, in addition to intrinsic vasculopathy, relatively fast and excessive growth (which outgrows its blood supply) in macro adenomas lead to ischemic necrosis followed by hemorrhage (1). Apoplectic episode results in a rapid increase in the in intrasellar pressure causing most of the clinical manifestations (Figure 1) of this syndrome (41).
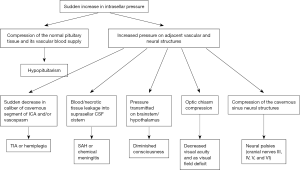
Imaging appearance of PA
Plenty of clinical series on PA have been reported, with emphasis on clinical diagnosis and management, and less emphasis on imaging features. Because of the potential grave prognosis of PA, radiologists should be familiar with its imaging characteristics and other acute clinical scenarios with similar clinical and/or imaging findings as PA.
Computed tomography (CT)
Usually almost all the patients initially undergo emergency CT imaging based on symptoms related to PA, though clinical suspicion might be different. Acute hemorrhagic infarct of the pituitary gland may be seen on CT as a large heterogeneously hyper dense sellar mass which may have a supra-sellar extension. Non-hemorrhagic infarct will usually show no abnormalities without intravenous contrast. Following intravenous contrast rim enhancement is suggestive but not diagnostic of apoplexy (3,22,42,43). In non-hemorrhagic PA or without a pre-existing pituitary adenoma, CT examination may be non- specific. Even though CT can rule out other diseases such as SAH or craniopharyngioma, an MR examination can better characterize a suspected PA (22,44).
Magnetic resonance imaging (MRI)
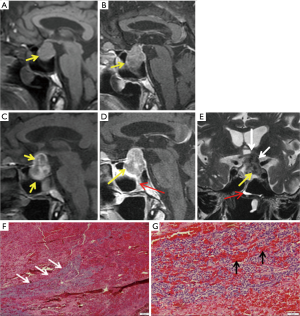
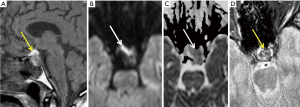
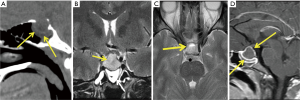
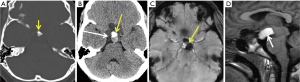
MRI appearance of the sellar/suprasellar mass depends on the time of onset and whether hemorrhage is present (Figures 2,3) or not (Figures 4,5). In hemorrhagic lesions, both T1 and T2 weighted images will initially show low signal, however, subacute hemorrhage will become hyper intense (Table 2). T2 weighted images are also useful in the evaluation of potential compressions of the optic chiasm (Figure 2E) and the hypothalamus (45). Following intravenous contrast, the most common finding in both hemorrhagic and non-hemorrhagic infarct (Figure 5) is peripheral rim enhancement in acute phase. The surrounding dura may show reactive thickening and enhancement. In the acute phase, both hemorrhagic and non-hemorrhagic apoplexy will show high signal on diffusion weighted imaging with the corresponding low signal on apparent diffusion coefficient (ADC) maps corresponding to area of infract (Figure 3B,C) (44,45). Gradient echo (GE) or susceptibility (T2*) weighted images can differentiate between these, as hemorrhagic apoplexy will show blooming (Figure 3D) within the sellar due to paramagnetic effects of bleeding products (deoxy-Hb and met-Hb) (46).
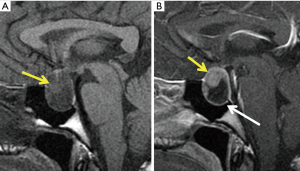
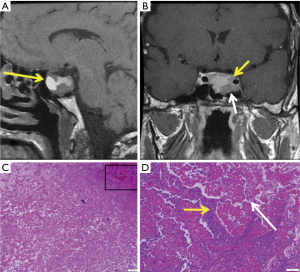

Full table
Many authors (22,47,48) have described a specific sign of hemorrhagic PA: fluid debris level within the mass. The T1 hyper intense upper fluid corresponds to free extracellular meta-Hb and the T1 iso to hypo intense lower layer related to the sediment of blood products of late subacute hemorrhage (47,48). Thickening of sphenoid sinus mucosa (Figure 2D,E) is another important specific MR finding of the acute phase of hemorrhage which is highly suggestive of PA (22,49). Mucosal thickening is probably related to venous congestion in this region and resolves later spontaneously (22). MRI is much superior to CT in the diagnosis of PA with a sensitivity ranging from 88% to 90% (22,49). PA features can be accurately predicted from MR imaging and correlate with the pathologic reports and operative findings (22).
Clinical mimics
The clinical syndrome of headache and visual changes that can mimic PA, has a myriad of causes, which often prompts the need for CT and/or MR imaging in the acute setting.
Pregnancy hyperplasia
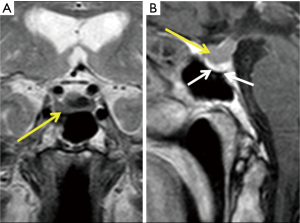
Pregnancy and the postpartum state may induce a number of pituitary related disorders due to the changes in systemic hormone levels. During pregnancy there is progressive enlargement of the pituitary gland to at least double the size and peaks in the early postpartum period; however, the vertical height should remain at 12 mm or below. Normal appearance of the gland is typically seen in 6 months post-partum (50). Patient may present with sudden onset headache with or without visual field deficits and can have acute hypopituitarism.
Imaging
Homogeneous signal characteristics within an enlarged pituitary gland (<12 mm) with superior convexity, without focal hyper intense signal on T1 or sellar blooming on T2* gradient echo image to suggest hemorrhage (Figure 6). Intravenous contrast administration should be avoided in pregnancy.
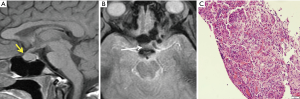
Pituitary metastasis
The sellar is relatively common location for metastatic deposits in autopsies of cancer patients however lesions are usually asymptomatic and not visible by imaging. The most common primary tumor types are lung (24.2–36%) and breast (33–37.2%) (51,52). Sellar metastases sometime clinically mimic PA as patients may present with acute onset double vision and headache.
Imaging
T1 weighted image following intravenous contrast at the time of acute presentation may show a heterogeneous enhancing sellar mass with or without suprasellar extension and minimal mass effect upon the chiasm (Figure 7). Patients typically have metastatic disease elsewhere (lung, breast) (Figure 7). Metastases may extend laterally into the cavernous sinus and superiorly into the pituitary stalk, hypothalamus, and optic chiasm (52).
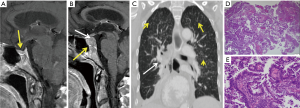
Pituitary abscess
Infection of the pituitary with abscess formation is a rare cause of a sellar or suprasellar mass and suspected to comprise less than 1% of all sellar lesions (19,53). Similar to other intracranial abscesses, pituitary abscess can occur from hematogenous seeding or direct extension from adjacent diseases such as sinusitis or meningitis. Less common causes include a contaminated cerebrospinal fluid fistula and cavernous sinus thrombophlebitis. The pre-existing pituitary lesion, prior surgery, and immunosuppression are the most common risk factors (53,54).
Imaging
Features are nonspecific and they may show varying degrees of T1 and T2 signal depending on the presence of coexisting proteinaceous fluid and hemorrhage (Figure 8A-C). Enhancement patterns may also vary however a ring or peripheral enhancement pattern (Figure 8D) has been described (53,54). Similar to PA, pituitary abscess also demonstrates restricted diffusion. However, unlike PA where restricted diffusion correspond to the area of infarct, restricted diffusion in pituitary abscess corresponds to central abscess cavity containing inflammatory cells, debris and macromolecules such as fibrinogen (54).
Craniopharyngioma
Craniopharyngioma is a WHO grade 1 neoplasm which arises from the epithelium of the Rathke’s pouch. It has bimodal occurrence. The adamantinomatous type is the common pediatric brain tumor aside from glial tumors. Patients typically present with an acute headache and vision changes with previously known or unknown longstanding anterior and posterior pituitary hormone deficiency (45,55).
Imaging
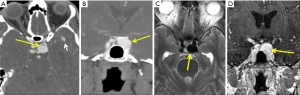
Approximately 75% of craniopharyngioma are suprasellar, 20% supra and intrasellar (20%) and only 5% are intra sellar in location. CT may show a mixed cystic and solid lesion with calcifications (Figure 9A,B). Areas of blooming on T2* weighted MR image (Figure 9C) corresponds to calcifications seen on CT (45,55). MR appearance may vary based on the amount of protein contents in the cyst fluid; however, is classically hyper intense on both T1 and T2 weighted images (Figure 9D). The solid portions enhance similarly to CT.
RCC
RCCs are ectodermal cysts which arise from the remnants of Rathke’s pouch. Unlike craniopharyngiomas, these are intrasellar cysts with suprasellar extension and usually located along the midline. A large sellar/suprasellar RCC may mimic PA clinically, with acute onset of headache and visual deficit (56).
Imaging
CT density characteristic is determined by the variable protein contents of the fluid. Unlike craniopharyngioma, calcification is rare and there is no solid enhancement. On MR, T1 hyper intensity related to variable protein contents (45,56). A typical T2 hypo-intense intra-cystic nodule is related to proteinaceous concretion within the RCC (Figure 10A). Unlike PA, does not present a fluid debris level (45). A claw sign of normal pituitary, however, can be seen draped around the cyst (Figure 10B).
Sellar aneurysm
Aneurysms from the cavernous segment of internal carotid artery, anterior or posterior communicating artery may extend into the sellar or suprasellar space (57-59). The aneurysm itself may cause headaches, however patients with thrombosis or rupture will report acute pain.
Imaging
Partially or completely thrombosed aneurysm in the sellar region can show high signal intensity on T1WI (57) and central high signal with hypo intense rim on T2WI. Aneurysms with repeat/recurrent hemorrhage and thrombosis may acquire a heterogeneous “onion-skin” appearance. CT angiography (Figure 11A,B) or DSA (digital subtraction angiography) is required to confirm diagnosis (57,58). If there is no thrombosis, the aneurysm will appear as a flow void on T2WI, occupying the sellar region (Figure 11C) and may demonstrate homogeneous filling of the aneurysm with contrast on MR angiography (Figure 11D).
Management of PA
A multidisciplinary team including endocrinologist, neurosurgeon, intensivist and neuro-ophthalmologist are required for the optimal care of PA with a proper management strategy that depends on the clinical manifestations. In patients with severe or progressive impairment of the consciousness, visual acuity or the visual fields, prompt surgical decompression may lead to the neurological or visual recovery in most of the cases (43). Patients with mild, stable clinical condition including those with isolated ocular palsies can be managed conservatively with stress doses of steroids and support of fluid and electrolyte balance in most cases (60). Frequent reassessment in ICU setting is mandatory because clinical conditions can deteriorate unpredictably (60). In that case, timely planned surgery can be beneficial, especially in terms of visual outcome. Irrespective of the treatment option, the pituitary hormonal function is less favorable with many patients remaining on long-term hormone replacement therapy (60). However, clear proof of optimal outcomes in the form of randomized controlled trials is lacking.
Role of imaging in therapy and follow-up
The role of imaging in the decision-making process for a proper therapy is still debatable as surgical or conservative treatment decisions are made on the basis of severity of the clinical condition (60). However, serial MRI can predict the evolution of PA in patients with mild symptoms who can be safely managed conservatively (43). Recurrent PA has been reported with both conservative and surgical treatment approaches, without a significant incidence difference between these groups (43,61). The pre-existing adenoma may regrow after PA and then might re-bleed (42). In those patients treated conservatively, an incidence of tumor regrowth after bleeding ranges from 6% to 90% that necessitates the need of clinical and radiological follow-up 3–6 months after PA and every year for at least 5 years (43,61).
Conclusions
PA is a potentially life threatening medical emergency and imaging is important to rule out other clinical mimics. Though, CT imaging is the most common initial study during the acute onset PA symptoms, MRI having better sensitivity should always be performed in acute and subacute phase of PA. Although MRI can play a crucial role in the conservative management, more studies are still needed to define its role in decision-making between surgical vs. conservative management. MRI should be considered in the follow-up of these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy: its incidence and clinical significance. J Neurosurg 1981;55:187-93. [Crossref] [PubMed]
- Cardoso ER, Peterson EW. Pituitary apoplexy: a review. Neurosurgery 1984;14:363-73. [Crossref] [PubMed]
- Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg 2007;109:63-70. [Crossref] [PubMed]
- Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med 2008;23:75-90. [Crossref] [PubMed]
- Bonicki W, Kasperlik-Zaluska A, Koszewski W, Zgliczynski W, Wislawski J. Pituitary apoplexy: endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir 1993;120:118-22. [Crossref] [PubMed]
- Mortimer AM, Bradley MD, Stoodley NG, Renowden SA. Thunderclap headache: diagnostic considerations and neuroimaging features. Clin Radiol 2013;68:e101-13. [Crossref] [PubMed]
- Ebersold MJ, Laws ER, Scheithauer BW, Randall RV. Pituitary apoplexy treated by transsphenoidal surgery. A clinicopathological and immunocytochemical study. J Neurosurg 1983;58:315-20. [Crossref] [PubMed]
- Mohr G, Hardy J. Haemorrhage, necrosis and apoplexy in pituitary adenomas. Surg Neurol 1982;18:181-9. [Crossref] [PubMed]
- Fernández-Balsells MM, Murad MH, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, Lampropulos JF, Natividad I, Perestelo-Pérez L, Ponce de León-Lovatón PG, Erwin PJ, Carey J, Montori VM. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab 2011;96:905-12. [Crossref] [PubMed]
- Nielsen EH, Lindholm J, Bjerre P, Christiansen JS, Hagen C, Juul S, Jørgensen J, Kruse A, Laurberg P. Frequent occurrence of pituitary apoplexy in patients with nonfunctioning pituitary adenoma. Clin Endocrinol (Oxf) 2006;64:319-22. [Crossref] [PubMed]
- Randall BR, Couldwell WT. Apoplexy in pituitary microadenomas. Acta Neurochir (Wien) 2010;152:1737-40. [Crossref] [PubMed]
- Jankoswski PP, Crawford JR, Khanna P, Malicki DM, Ciacci JD, Levy ML. Pituitary tumor apoplexy in adolescentes. Word Neurosurg 2015;83:644-51. [Crossref]
- Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy: presentation, surgical management and outcome in 21 patients. Neurosurgery 1990;26:980-6. [Crossref] [PubMed]
- Rangel-Castilla L, Ríos-Alanis M, Torres-Corzo J, Rodríguez-Della-Vechia R, Chavez-López R. Pituitary apoplexy as the presenting symptom of a recurrent craniopharyngioma. Rev Neurol 2004;39:297-8. [PubMed]
- Kim E. A rathke's cleft cyst presenting with apoplexy. J Korean Neurosurg Soc 2012;52:404-6. [Crossref] [PubMed]
- Verma R, Patil TB, Lalla R. Pituitary apoplexy syndrome as the manifestation of intracranial tuberculoma. BMJ Case Rep 2014;2014:bcr2013201272. [Crossref] [PubMed]
- Dan NG, Feiner RI, Houang MT, Turner JJ. Pituitary apoplexy in association with lymphocytic hypophysitis. J Clin Neurosci 2002;9:577-80. [Crossref] [PubMed]
- Husain Q, Zouzias A, Kanumuri VV, Eloy JA, Liu JK. Idiopathic granulomatous hypophysitis presenting as pituitary apoplexy. J Clin Neurosci 2014;21:510-2. [Crossref] [PubMed]
- Kingdon CC, Sidhu PS, Cohen J. Pituitary apoplexy secondary to an underlying abscess. J Infect 1996;33:53-5. [Crossref] [PubMed]
- Chhiber SS, Bhat AR, Khan SH, Wani MA, Ramzan AU, Kirmani AR, Malik NK, Wani AA, Rather T. Apoplexy in sellar metastasis: a case report and review of literature. Turk Neurosurg 2011;21:230-4. [PubMed]
- Sheehan HL, Stanfor JP. The pathogenesis of postpartum pituitary necrosis of the anterior lobe of the pituitary gland. Acta Endocrinol 1961;37:479-510.
- Semple PL, Jane JA, Lopes MBS, Laws ER. Pituitary apoplexy: correlation between magnetic resonance imaging and histopathological results. J Neurosurg 2008;108:909-15. [Crossref] [PubMed]
- Gorczyca W, Hardy J. Microadenomas of the human pituitary and their vascularization. Neurosurgery 1988;22:1-6. [Crossref] [PubMed]
- Kruse A, Astrup J, Cold GE, Hansen HH. Pressure and blood flow in pituitary adenomas measured during transsphenoidal surgery. Br J Neurosurg 1992;6:333-41. [Crossref] [PubMed]
- Möller-Goede DL, Brändle M, Landau K, Bernays RL, Schmid C. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol 2011;164:37-43. [Crossref] [PubMed]
- Doglietto F, Costi E, Villaret AB, Mardighian D, Fontanella MM, Giustina A. New oral anticoagulants and pituitary apoplexy. Pituitary 2016;19:232-4. [Crossref] [PubMed]
- Carija R, Vucina D. Frequency of pituitary tumor apoplexy during treatment of prolactinomas with dopamine agonists: a systematic review. CNS Neurol Disord Drug Targets 2012;11:1012-4. [Crossref] [PubMed]
- Chng E, Dalan R. Pituitary apoplexy associated with cabergoline therapy. J Clin Neurosci 2013;20:1637-43. [Crossref] [PubMed]
- Louwerens M, de Herder WW, Postema PT, Tanghe HL, Lamberts SW. Pituitary insufficiency and regression of acromegaly caused by pituitary apoplexy following cerebral angiography. Eur J Endocrinol 1996;134:737-40. [Crossref] [PubMed]
- Reichenthal E, Manor RS, Shalit MN. Pituitary apoplexy during carotid angiography. Acta Neurochirurgica 1980;54:251-5. [Crossref] [PubMed]
- Alzetani A, Fisher C, Costa R, Ohri SK. Ptosis post cardiac surgery: a case of pituitary apoplexy. Ann Thorac Surg 2002;73:300-1. [Crossref] [PubMed]
- Liberale G, Bruninx G, Vanderkelen B, Dubois E, Vandueren E, Verhelst G. Pituitary apoplexy after aortic abdominal aneurysm surgery: a case report. Acta Chirurgica Belgica 2006;106:77-80. [Crossref] [PubMed]
- Koga T, Miyao M, Sato M, Hirota K, Kakuyama M, Tanabe H, Fukuda K. Pituitary apoplexy during general anesthesia in beach chair position for shoulder joint arthroplasty. J Anesth 2010;24:476-8. [Crossref] [PubMed]
- Uchiyama H, Nishizawa S, Satoh A, Yokoyama T, Uemura K. Post-traumatic pituitary apoplexy - two case reports. Neurol Med Chir (Tokyo) 1999;39:36-9. [Crossref] [PubMed]
- Yamamoto T, Yano S, Kuroda J, Hasegawa Y, Hide T, Kuratsu J. Pituitary apoplexy associated with endocrine stimulation test: endocrine stimulation test, treatment, and outcome. Case Rep Endocrinol 2012;2012:826901. [Crossref] [PubMed]
- Weisberg LA. Pituitary apoplexy. Association of degenerative change in pituitary adenoma with radiotherapy and detection by cerebral computed tomography. Am J Med 1977;63:109-15. [Crossref] [PubMed]
- Xekouki P, Mastroyiannis SA, Avgeropoulos D, de la Luz Sierra M, Trivellin G, Gourgari EA, Lyssikatos C, Quezado M, Patronas N, Kanaka-Gantenbein C, Chrousos GP, Stratakis CA. Familial pituitary apoplexy as the only presentation of a novel AIP mutation. Endocrine-Related Cancer 2013;20:L11-4. [Crossref] [PubMed]
- Schechter J. Ultrastructural changes in the capillary bed of human pituitary tumors. Am J Pathol 1972;67:109-26. [PubMed]
- Pergolizzi RS Jr, Nabavi A, Schwartz RB, Hsu L, Wong TZ, Martin C, Black PM, Jolesz FA. Intra-operative MR guidance during transsphenoidal pituitary resection: preliminary results. J Magn Reson Imaging 2001;13:136-41. [Crossref] [PubMed]
- Bonneville JF, Bonneville F, Cattin F. Magnetic resonance imaging of pituitary adenomas. Eur Radiol 2005;15:543-8. [Crossref] [PubMed]
- Zayour DH, Selman WR, Arafah BM. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: relation to pituitary function. J Clin Endocrinol Metab 2004;89:5649-54. [Crossref] [PubMed]
- Randeva HS, Schoebel J, Byrne J, Esiri M, Adams C, Wass J. Classical pituitary apoplexy: clinical features, management and outcome. Clin. Endocrinol (Oxf) 1999;51:181-8. [Crossref] [PubMed]
- Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJ. Acute management of pituitary apoplexy—surgery or conservative management? Clin Endocrinol (Oxf) 2004;61:747-52. [Crossref] [PubMed]
- Rogg JM, Tung GA, Anderson G, Cortez S. Pituitary apoplexy: early detection with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2002;23:1240-5. [PubMed]
- Osborn AG. Pituitary apoplexy. In: Osborn A, Salzman KL, Barkovich AJ. editors. Diagnostic imaging. Salt Lake City: Amirsys Inc, 1972.
- Tosaka M, Sato N, Hirato J, Fujimaki H, Yamaguchi R, Kohga H, Hashimoto K, Yamada M, Mori M, Saito N, Yoshimoto Y. Assessment of hemorrhage in pituitary macroadenoma by T2*-weighted gradient-echo MR imaging. Am J Neuroradiol 2007;28:2023-9. [Crossref] [PubMed]
- Piotin M, Tampieri D, Rüfenacht DA, Mohr G, Garant M, Del Carpio R, Robert F, Delavelle J, Melanson D. The various MRI patterns of pituitary apoplexy. Eur Radiol 1999;9:918-23. [Crossref] [PubMed]
- Kurihara N, Takahashi S, Higano S, Ikeda H, Mugikura S, Singh LN, Furuta S, Tamura H, Ishibashi T, Maruoka S, Yamada S. Haemorrhage in pituitary adenoma: correlation of MR imaging with operative findings. Eur Radiol 1998;8:971-6. [Crossref] [PubMed]
- Arita K, Kurisu K, Tominaga A, Sugiyama K, Ikawa F, Yoshioka H, Sumida M, Kanou Y, Yajin K, Ogawa R. Thickening of sphenoid sinus mucosa during the acute stage of pituitary apoplexy. J Neurosurg 2001;95:897-901. [Crossref] [PubMed]
- Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F. Pregnancy and pituitary disorders. Eur J Endocrinol 2010;162:453-75. [Crossref] [PubMed]
- Morita A, Meyer FB, Laws ER. Jr Symptomatic pituitary metastases. J Neurosurg 1998;89:69-73. [Crossref] [PubMed]
- He W, Chen F, Dalm B, Kirby PA, Greenlee JD. Metastatic involvement of the pituitary gland: A systematic review with pooled individual patient data analysis. Pituitary 2015;18:159-68. [Crossref] [PubMed]
- Vates GE, Berger MS, Wilson CB. Diagnosis and management of pituitary abscess: a review of twenty-four cases. J Neurosurg 2001;95:233-41. [Crossref] [PubMed]
- Takao H, Doi I, Watanabe T. Diffusion-weighted magnetic resonance imaging in pituitary abscess. J Comput Assist Tomogr 2006;30:514-6. [Crossref] [PubMed]
- Pusey E, Kortman KE, Flannigan BD, Tsuruda J, Bradley WG. MR of craniopharyngiomas: tumor delineation and characterization. AJR Am J Roentgenol 1987;149:383-8. [Crossref] [PubMed]
- Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT. Hemorrhagic and nonhemorrhagic Rethke cleft cysts mimicking pituitary apoplexy. J Neurosurg 2008;108:3-8. [Crossref] [PubMed]
- Bonneville F, Cattin F, Marsot-Dupuch K, Dormont D, Bonneville JF, Chiras J. T1 signal hyperintensity in the sellar region: spectrum of findings. Radiographics 2006;26:93-113. [Crossref] [PubMed]
- Xu K, Yuan Y, Zhou J, Yu J. Pituitary adenoma apoplexy caused by rupture of an anterior communicating artery aneurysm: case report and literature review. World J Surg Oncol 2015;13:228. [Crossref] [PubMed]
- Bonfield CM, Gardner PA. Posterior communicating artery aneurysm rupture mimicking apoplexy. Surg Neurol Int 2011;2:169. [Crossref] [PubMed]
- Rajasekaran S, Vanderpump M, Baldeweg S. UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf) 2011;74:9-20. [Crossref] [PubMed]
- Gruber A, Clayton J, Kumar S, Robertson I, Howlett TA, Mansell P. Pituitary apoplexy: retrospective review of 30 patients–is surgical intervention always necessary? Br J Neurosurg 2006;20:379-85. [Crossref] [PubMed]


