Case-based approach to demonstrate utility of cardiac magnetic resonance imaging (MRI) for planning biventricular repair with inconclusive echo: illustration of two cases
Introduction
Congenital heart defects that present earlier in life and involving a hypoplastic ventricle are sometimes channeled towards single-ventricle repair because of anatomical or logistic challenges (1). With the single-ventricular repair, the final result is the shunting of venous return directly into the pulmonary artery and the utilization of the functioning ventricle for systemic circulation (2). Though single-ventricular repair has remarkable short-term results, long-term survivors experience declines in exercise tolerance, heart failure, arrhythmias, and thromboembolic complications (3). Given long-term functional and survival advantages of a two-ventricle circulation, exploring feasibility of biventricular repair is essential for improving prognosis. However, a successful biventricular repair requires sufficiently functioning ventricles to sustain a balanced flow through the pulmonary and systemic circuit. To evaluate the functional adequacy of the ventricle with hypoplasia, echocardiogram is conventionally used. Echocardiography can be utilized to assess systolic function through measured fractional shortening and diastolic function through Doppler-determined velocities across atrioventricular valves (4). However, echocardiogram assessment suffers from some shortcomings. For example, the fractional shortening, calculated by the end diastolic dimension minus the end systolic dimension divided by the end diastolic dimension, is affected by the preload size and relies on standard geometrical assumptions (5). On the other hand, electrocardiography-gated 2-dimensional steady state free precession (2D SSFP) cine cardiac magnetic resonance imaging (MRI) can better assess ventricular function by measuring ventricular volumes more precisely and by quantifying the ejection fraction (4). In this report, we demonstrate how cardiac MRI can help steer the cardiac surgeon’s decision towards biventricular repair when echocardiogram is inconclusive.
Case presentation
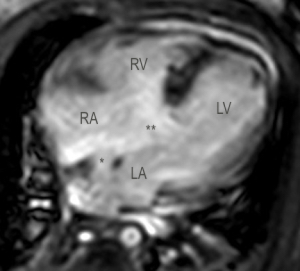
Case 1, a 2-week-old girl with Trisomy 21 demonstrated a complete atrioventricular canal defect with moderate right ventricular hypoplasia on initial echocardiogram. In atrioventricular canal defects with borderline or hypoplastic development of one of the ventricles, complete biventricular repair might be challenging or prohibitory. Furthermore, Trisomy 21 syndrome adds a significant morbid factor in patients where single-ventricle palliation or 1 and ½ ventricle repair is to be pursued. However, cardiac MRI was utilized to further evaluate the patient’s cardiac anatomy/function and the patient’s candidacy for biventricular repair. MRI showed left ventricular stroke volume and index as 5.3 mL and 23 mL/m2 and 4.4 mL and 19.2 mL/m2 for the right ventricle respectively (Figure 1). These MRI findings showing adequate ventricular development were utilized to steer the cardiac surgeon’s decision towards biventricular repair in which a modified single patch approach was elected to septate this atrioventricular canal defect using autologous pericardium while tricuspid and mitral valve annuloplasty were performed to downsize the annulus to maximum diameters of 13 and 12 mm, respectively. The patient’s 6-month evaluation showed great recovery, with appropriate growth of right ventricle and was managed with sildenafil for the expected borderline elevated pulmonary vascular resistance.
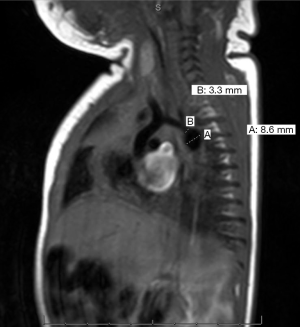
Case 2, a 2-week-old boy presented with a juxtaductal coarctation of the aorta and hypoplastic left heart syndrome (hypoplastic mitral valve z-score, −2.5; aortic valve z-score, −3.1; and left ventricular internal dimension-diastole z-score, −3.05) as seen on neonatal echocardiogram. The patent ductus arteriosus was maintained with prostaglandin E 0.03 mcg/kg/min while consideration for a single-ventricular repair was contemplated. However, after close monitoring for 10 days, cardiac MRI was performed to further examine the development of the left ventricle and its valves. MRI demonstrated the juxtaductal coarctation, hypoplastic mid-aortic arch, and normal left ventricular stroke volume and index of 3.6 mL and 23.4 mL/m2, respectively (Figure 2). At the aortic valve, the stroke volume is 2.5 mL with a regurgitation fraction of 5.8%. These MRI findings confirmed adequate inflow through the mitral valve, stable development of the borderline left ventricle, and suitable deliverance of the cardiac output through the aortic valve without evidence of stenosis. In this case, qualitative and quantitative echocardiographic and MRI data supported our biventricular strategy towards left ventricular rehabilitation to sustain adequate cardiac output based on growth of left heart structures and volumes. The patient’s 1-year follow-up exhibited normal left and right ventricular size and function with moderate mitral valve stenosis and growth to 9.19 kg (35th percentile) from 3 kg at birth.
Discussion
Cardiac MRI complements echocardiography by offering additional analytical information in assessing cardiac function. In particular, cardiac MRI provides high contrast between blood and adjacent structures in multiple planes (orthogonal, four-chamber, two-chamber, ventricular short axis and oblique outflow planes) (1,6). The diverse planes allow the clinician to better view the cardiac valves and ventricles in patients with complex intracardiac anatomy and from views not conventionally obtained in echocardiography. Additionally, flow from regurgitation and altered flow due to stenosis can be visualized through flow-related signal loss on cardiac MRI (6). This can be used to assess the valve’s form and visualize lesions. Furthermore, cardiac MRI delivers the most accurate assessment of left and right ventricular volumes, mass, and ejection fraction, which are valuable in determining ventricular development and cardiac function (7). In addition, cardiac MRI calculates ventricular stroke volume, which in conjunction with ventricular volume can be used to quantify valve regurgitation.
Cardiac MRI’s role in surgical planning often arises when echocardiogram provided inadequate information due to poor transthoracic ultrasound signals, difficult cardiac anatomy, and borderline assessment of ventricular volume and function (1). In particular with our cases, we were interested in the cardiac MRI’s more accurate assessment of ventricular stroke volume, ventricular stroke index per body surface area, and valve regurgitation. With a complete atrioventricular canal defect as seen in our case 1, the objective was to determine whether there is a balanced flow through the common valve between the left and right ventricles to support separate pulmonary and systemic circuits in a biventricular circulation. With cardiac MRI, we looked at the stroke index per body surface area of the right and left ventricles and both exhibited adequate flow and a small difference between them. With the hypoplastic left heart syndrome in our case 2, the objective was to quantify the left ventricular stroke volume and index per body surface area given the borderline assessment based on the echocardiogram. Feasibility of biventricular repair remains a complex issue in neonates with critically underdeveloped left heart structures. Left ventricular moderate hypoplasia represents a clinical decision-management trivia, due to the pressing issue of an early decision to pursue biventricular or univentricular strategy, often, just within the first week of life. An early decision towards biventricular approach, although intuitively favorable, may be associated with detrimental outcome if pursued in an unsuitable patient. A left ventricular end-diastolic volume (LVEDV) by MRI of 20 mL/m2 or more is sufficient to support biventricular repair, especially in light of the fact that echocardiographically obtained volumes can underestimate left ventricular critical dimensions. In our case, we documented adequate preload though the systemic atrioventricular valve which was indicative of future restoration of normal left ventricular size and cardiac output with the least morbidity. Because in both cases echocardiogram demonstrated borderline functional assessment, the use of cardiac MRI to more precisely quantify cardiac functions truly facilitated in convincing the surgeon that biventricular repair would be better indicated than single-ventricular repair. Future assessment of such cases may involve 4-dimensional flow cardiac magnetic resonance in which 3-dimensional imaging is resolved with a time dimension. Such techniques offer the potential for retrospective flow volume measurements at any theoretical plane and more consistent quantifications (8).
In conclusion, paired MRI and echocardiographic volumetric-flow variables obtained during the early neonatal period is a feasible approach and advocated in borderline left heart structures. Accuracy of left ventricular volumes and indices by echocardiography can be supported by a dynamic interplay that advanced imaging anatomic and flow-kinetics assessment offers and may effectively navigate decision-making process towards a sustainable biventricular path in properly selected cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: This article was presented as poster at Society for Cardiovascular Magnetic Resonance 2017. February 1–4, 2017; Washington, DC, USA.
Informed Consent: Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kottayil BP, Sunil GS, Kappanayil M, Mohanty SH, Francis E, Vaidyanathan B, Balachandran R, Nair SG, Kumar RK. Two-ventricle repair for complex congenital heart defects palliated towards single-ventricle repair. Interact Cardiovasc Thorac Surg 2014;18:266-71. [Crossref] [PubMed]
- Nayak S, Booker PD. The Fontan circulation. Contin Educ Anaesth Crit Care Pain 2008;8:26-30. [Crossref]
- de Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol 2010;7:520-7. [Crossref] [PubMed]
- Cheung YF. Functional assessment for congenital heart disease. Korean Circ J 2014;44:59-73. [Crossref] [PubMed]
- Koestenberger M. Transthoracic echocardiography in children and young adults with congenital heart disease. ISRN Pediatr 2012;2012:753481. [Crossref] [PubMed]
- Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:7. [Crossref] [PubMed]
- Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387-96. [Crossref] [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72. [Crossref] [PubMed]


