Diagnostic contribution of magnetic resonance imaging in an atypical presentation of motor neuron disease
Introduction
Motor neuron disease (MND) is a neurodegenerative disease with typical onset between the 6th and 7th decade, determining progressive and relentless motor deterioration involving both upper and lower motor neurons (UMN and LMN). Several variants at onset are recognized, aside from the “classic” or “spinal” variant, that may display, especially at the very beginning, clinical features indicative of exclusive UMN or LMN involvement—such as “monomelic” or “flail arm” presentations, known to be associated with a relatively longer disease duration (1). At the opposite end of the amyotrophic lateral sclerosis (ALS) clinical spectrum there are primary lateral sclerosis (PLS) and progressive muscular atrophy (PMA), characterized by an earlier age of onset, a significantly longer average survival, and by degeneration of only UMNs or LMNs, respectively, during the entire disease course (2,3). PLS usually begins as a spastic paraparesis displaying an ascending symmetrical pattern of progression, although asymmetrical, multifocal or pseudobulbar presentations have been reported. The absence of development of LMN signs after 4 years of onset allows to classify the disease as PLS rather than classical ALS (4).
An isolated pyramidal syndrome, with complete absence of LMN signs, is a rare phenotype in the context of MND (less than 4% of total cases), especially if restricted to only one limb (5). It may raise the suspect of a MND at the very beginning, especially if presenting in an insidious way, but it is essential to exclude other more likely diagnostic possibilities (i.e., lesional causes) and to consider the progression of the syndrome over time. The role of MR in confirming an extensive UMN involvement of possible degenerative etiology has been highlighted by previous works (6-8). Moreover, the evaluation of white matter abnormalities by diffusion tensor imaging (DTI) proved useful for distinguishing between ALS and PLS (9,10). Here we describe a case of MND presenting as pure spastic monoparesis in which advanced MR techniques gave a substantial contribution in confirming the diagnosis and assessing the severity of UMN involvement.
Case presentation
An 81-year-old woman presented a left upper limb weakness with subacute (18 months) onset, which spread from the hand to the whole arm. She also experienced progressive stiffness and clumsiness in fine movements, eventually leading to severe functional impairment. Neurological examination showed moderate-severe spastic monoparesis of the left upper limb with pathological hyperreflexia and Hoffmann, Troëmner and Bechterew signs. She presented an almost complete paralysis of all left hand movements. There was only mild distal muscular atrophy, while fasciculations were absent. The contralateral upper limb and the lower limbs displayed normal strength, tone and reflexes. No pyramidal signs in the other districts, nor bulbar or respiratory involvement were present. There were no superficial or deep sensory disturbances in the whole body. Neuropsychological assessment showed normal cognitive functions. She denied familiarity for neurological disorders and her past medical history included achalasia, erythema nodosum and cervical spondyloarthrosis. Extensive laboratory analyses, including endocrine and autoimmune evaluations, screening for neoplastic markers, anti-ganglioside and anti-neuronal antibodies were not contributive. The only significant alterations were the following: ESR (first hour) 80 mm, CRP 1.08 mg/dL, fibrinogen 683 mg/dL, ferritin 739 ng/mL. Serum CK was equal to 31 U/L. Anti-nuclear antibodies were present at high titer (1:640) with homogeneous pattern. No anti-ENA was detected.
Motor and sensory nerve conduction study was normal. No denervation was found in the examined muscles (deltoid, first dorsal interosseous and biceps) in the left upper arm. Motor unit potential showed normal amplitude and duration with diffuse reduction of recruitment. A Magstim 200 stimulator was used to deliver transcranial and paravertebral stimuli through a circular coil. Motor evoked potentials were recorded bilaterally from biceps brachii and abductor digiti minimi. Central motor conduction time (CMCT) was calculated by subtracting the peripheral conduction time from spinal cord to muscles (peripheral motor latency, PML) from the latency of responses evoked by cortical stimulation (central motor latency, CML). We found no cortical response recording from left abductor digiti minimi and a pathological lengthening of CMCT recording from left biceps brachii, measuring 23.75 msec (with normal PML, 5 msec, and markedly prolonged CML, 28.75 ms).
The patient underwent brain and spine MR assessment on a 3 Tesla scanner (Trio, Siemens Medical Systems, Erlangen, Germany). The acquisition protocol included, beside conventional sequences such as T2w or FLAIR, a structural T1w volumetric sequence used for co-registration with DTI for the evaluation of the corticospinal tract (CST) integrity, by means of tractography analysis, and magnetic resonance spectroscopy (MRS) at the level of the motor cortex (MC). Structural T1w volumes were acquired by three-dimensional Magnetization-Prepared RApid Gradient-Echo sequence (MPRAGE; TE =3.4 ms; TR =1900 ms; TI =900 ms; Flip Angle =9°, FOV =250; slice plane = axial; slice thickness =1 mm; voxel size =1×1×1 mm3; number of slices =160).
A 2D Multi-voxel MRS Spin Echo (point-resolved spectroscopy) sequence was acquired, with water suppression by means of selective excitation (TE: 270 ms, FOV 160×160 mm2, acquisition matrix 16×16, slice thickness: 15 mm, zero-filled to a voxel size of 5×5×15 mm3) as a single axial slice centered at the level of the “hand knobs” of the MC. Diffusion weighted images (DWI) were acquired with diffusion gradients oriented along 64 non-collinear directions (TR =5,200 ms, TE =82 ms, flip angle =90°, matrix 128×128, 3 mm thick axial slices, 8 B0 values with a diffusion weighting b =1,000 s/mm2). DTI data were preliminarily corrected for head movements and eddy currents distortions using Medical Image Processing, Analysis and Visualization software (MIPAV, http://mipav.cit.nih.gov/). Each diffusion-weighted volume was deformed to match the corresponding B0 volume using a cubic Lagrangian interpolation. Diffusion sensitizing gradient directions were corrected according to the corresponding deformation vectors and a diffusion-tensor model was fitted at each voxel using Diffusion Toolkit (http://trackvis.org/dtk), generating fractional anisotropy (FA), and mean diffusivity (MD) maps. Spectroscopy data were analyzed using the software available on the scanner (Syngo MR B17). Processing included Fourier transformation, Gaussian filtering in the time domain, and phase and baseline correction, followed by identification of the choline (Cho, centered at 3.22 ppm), creatine and phosphocreatine (Cr, 3.02 ppm), and N-Acetyl-Aspartate (NAA, 2.02 ppm) peaks, and fitting of the corresponding integrals under the curve. The ratio of NAA to Crea + Cho was used as marker of UMN loss/dysfunction. Both DTI and MRS results were compared to data obtained with the same analysis on a group of five age-matched healthy controls (HC).
Brain magnetic resonance imaging (MRI) showed prominent atrophy and T2-hypointensity of the right MC and milder atrophy of the post-central gyrus and of the entire parietal lobe, with no evidence of remarkable cerebrovascular disease along the pyramidal system, thus ruling out an ischemic etiology (Figure 1A,B). No signal abnormalities were present on the conventional sequences along the CST.
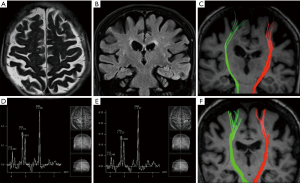
Compared to HC, DTI and MRS analysis showed impairment of both CST diffusion and MC metabolite concentration, respectively (Figure 1C-E), with a greater involvement of the right side. Namely, the DTI analysis, using ROIs placed in the subcortical white matter of the precentral gyrus, showed reduced FA and increased MD (0.51±0.12 and 858±111 mm2/s ×10−6, respectively) in the patient’s right CST compared to the left (0.55±0.13, and 806±108 mm2/s ×10−6, respectively), with marked asymmetry in terms of relative track counts (right = 33; left =74).
The corresponding DTI metrics in the HC group (Figure 1F) were: FA right: 0.54±0.14, left: 0.54±0.15; MD right: 748±102, left: 756±105 mm2/s ×10−6; track counts right: 165, left: 165.
At MRS, a diffuse reduction of the NAA/Cho + Cr ratio was present in both MC areas, more prominent on the right (0.97) compared to the contralateral side (1.03) (Figure 1D,E), the ratio being clearly lower than the corresponding values in the HC group (right =1.32±0.12; left =1.29±0.11).
Spine MRI disclosed non-significant spondyloarthrosis, without alterations in spinal cord morphology or signal. Therefore, DTI at this level was not performed.
Discussion
ALS or MND is the most frequent neurodegenerative disease involving both UMNs and LMNs. Its main clinical variant (spinal variant) is typically asymmetric and distal at the onset.
Several elements make our case an unusual presentation of MND: first, the late age of onset (8th decade), whereas sporadic MND classically begins between the 6th and the 7th decade of life and PLS even earlier (11). Another atypical aspect for MND is the subacute evolution of symptoms, which raised the suspicion of an ischemic or inflammatory, rather than degenerative, etiology. Also, the patient’s past medical history (achalasia, erythema nodosum), the increase of inflammatory indices (such as ESR, CRP, ferritin and fibrinogen) and the presence of ANA (antinuclear antibodies) at high titer, indicated the need of ruling out ischemic lesions of possible autoimmune origin. The third important point is the clinical phenotype displayed by our case, i.e., a spastic upper limb monoparesis. Among MND variants determining predominant upper limb impairment, monomelic and flail arm presentations mainly involve LMNs, with UMN signs possibly appearing during the disease course (12). Here, the clinical syndrome consisted of pure UMN features, and no LMN signs were present. Moreover, the electrophysiological examination completely excluded LMN involvement, and motor evoked potentials confirmed and underlined the damage along CST directed to the upper left limb, without appreciable alterations of central motor conduction to the other limbs. This “focal” impairment was again in favor of a lesional hypothesis (i.e., vascular or neoplastic), making MRI even more necessary. All similar cases reported in the literature were related to an ischemic etiology with acute onset. The only exception was a case of anti-phospholipid antibody syndrome presenting with a progressive monoparesis due to an ischemic lesion at the level of the precentral cortex (13). In our case, “conventional” MRI showed no vascular lesions that could explain the clinical features allowing us to exclude an ischemic etiology (14).
Therefore, we used advanced MR sequences in order to evaluate the whole CST, looking for focal or diffuse pathology along it. The DTI evaluation surprisingly evidenced bilateral impairment of CST diffusion metrics, with clear right-left asymmetry, pointing to a neurodegenerative etiology (15), which clinically appeared less likely at that time. Then, the marked reduction of NAA/Cho + Cr ratio in the MC shown by MRS further supported the hypothesis of UMN degeneration (6-8).
However, the diagnostic conclusions in our case are hampered by the atypical age and modality of onset, which still hinder a precise diagnostic definition either by considering it as an early phase of classical ALS or as a PLS. In fact, since the disease started no more than 18 months before our clinical evaluation, a clinically pure diagnosis of PLS is not possible yet (4). In conclusion, in this particular case of MND, whose nosographic framing has not been fully defined, advanced MRI techniques with DTI and MRS proved to be of great usefulness in confirming a diffuse UMN involvement, possibly at a more advanced stage than its clinical expression (16).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293-9. [Crossref] [PubMed]
- Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology 2009;72:1948-52. [Crossref] [PubMed]
- Kim WK, Liu X, Sandner J, Pasmantier M, Andrews J, Rowland LP, Mitsumoto H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology 2009;73:1686-92. [Crossref] [PubMed]
- Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, Rowland LP. The natural history of primary lateral sclerosis. Neurology 2006;66:647-53. [Crossref] [PubMed]
- Chiò A, Calvo A, Moglia C, Mazzini L, Mora G. PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 2011;82:740-6. [Crossref] [PubMed]
- Cervo A, Cocozza S, Saccà F, Giorgio SM, Morra VB, Tedeschi E, Marsili A, Vacca G, Palma V, Brunetti A, Quarantelli M. The combined use of conventional MRI and MR spectroscopic imaging increases the diagnostic accuracy in amyotrophic lateral sclerosis. Eur J Radiol 2015;84:151-7. [Crossref] [PubMed]
- Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M. Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol 2014;13:1228-40. [Crossref] [PubMed]
- Liu C, Jiang R, Yi X, Zhu W, Bu B. Role of diffusion tensor imaging or magnetic resonance spectroscopy in the diagnosis and disability assessment of amyotrophic lateral sclerosis. J Neurol Sci 2015;348:206-10. [Crossref] [PubMed]
- Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH, Matthews PM, Thompson AJ, Smith SM. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 2009;30:615-24. [Crossref] [PubMed]
- Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, Floeter MK. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 2011;134:2642-55. [Crossref] [PubMed]
- McGuire V, Nelson LM. Epidemiology of ALS. New York: Taylor & Francis, 2006.
- Agosta F, Al-Chalabi A, Filippi M, Hardiman O, Kaji R, Meininger V, Nakano I, Shaw P, Shefner J, van den Berg LH, Ludolph A. WFN Research Group on ALS/MND. The El Escorial criteria: strengths and weaknesses. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:1-7. [Crossref] [PubMed]
- Tsuda H, Tanaka K, Kishida S. Pure Motor Monoparesis in the Leg due to a Lateral Medullary Infarction. Case Rep Med 2012;2012:758482. [Crossref]
- Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L. Errors in neuroradiology. Radiol Med 2015;120:795-801. [Crossref] [PubMed]
- Woo JH, Wang S, Melhem ER, Gee JC, Cucchiara A, McCluskey L, Elman L. Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One 2014;9:e105753. [Crossref] [PubMed]
- Cocozza S, Russo C, Pontillo G, Ugga L, Macera A, Cervo A, De Liso M, Di Paolo N, Ginocchio MI, Giordano F, Leone G, Rusconi G, Stanzione A, Briganti F, Quarantelli M, Caranci F, D’Amico A, Elefante A, Tedeschi E, Brunetti A. Is advanced neuroimaging for neuroradiologists? A systematic review of the scientific literature of the last decade. Neuroradiology 2016;58:1233-9. [Crossref] [PubMed]


