Preliminary results of vibro-acoustography evaluation of bone surface and bone fracture
Introduction
Diagnostic ultrasonography is widely used in medicine to image internal structures in the body. The ultrasound penetration and image resolution are frequency-dependent, which impose a trade-off in the frequency selection. Thus, superficial structures such as muscles and tendons can be imaged at higher frequencies (7–18 MHz) with higher resolution than deeper structures such as liver and kidney, typically imaged at 1–6 MHz (1). In addition, the maximum response of quantitative ultrasound methods is usually associated with tissue resonance frequency, for example, soft tissues 30–400 Hz (2) and stiffer structures such as human dentin 0.5–1.4 MHz (3).
Vibro-acoustography (VA) is an imaging technique that combines multiple co-focused ultrasonic beams with different frequencies at the mega-hertz range to generate high spatial resolution focus of lower frequencies (hertz to kilo-hertz) (4,5). As a result, this technique allows access specific regions at different depths applying selected frequencies such that excited tissue will have maximum response. Assemblies using distinct types of transducers show that it is possible to obtain low frequency foci with lateral resolution of tenths of millimeters (6).
The nonlinear interaction between two ultrasonic beams of near frequencies, equal to ω = ω0 ± Δω/2 (being Δω the difference-frequency), gives rise to a pressure field with frequency Δω. The difference-frequency pressure field PΔω presents contributions from the parametric array (7), the interaction of sound-with-sound (4,7), and induced acoustic emission (8) by a time-modulated radiation force (9-12). The quantification of all contributions can be found in (13).
The VA signal is weighted by the mechanical properties of the sample, such as density, speed of sound, structural characteristics, and viscoelastic parameters. Clinical feasibility studies of VA were previously performed for detection of macrocalcifications in breast (14) and prostate (15). In addition, the use of fundamental frequencies (ω1 and ω2) with values lower than 1 MHz allows the generation of VA images of trabecular bone structures to aid in the diagnosis of osteoporosis (16). Using frequencies higher than 2 MHz, it is also possible to select superficial regions of bone structures and implants with generation of three-dimensional VA images to monitor postoperative total hip arthroplasty (17).
When a bone is fractured, its mechanical properties and vibrational characteristics (18) and, consequently, its acoustic scattering and resonant frequencies are changed. The aim of this study is to investigate the potential of VA imaging in the evaluation of bone surface and bone fracture. Preliminary VA images of excised fractured bones using high frequency primary beams are reported.
Methods
This pilot study was conducted using two fresh and cleaned chicken (Gallus gallus domesticus) femur bones. The approval of the Institutional Review Board was not necessary because no animal was sacrificed or submitted to experimental investigation. The bone samples were prepared removing the flesh from chicken thigh obtained in the supermarket. One femur was fractured in its distal region, resulting in a longitudinal separation with maximum cleft width of 2.5 mm. The other femoral bone was intact.
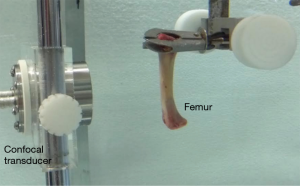
Images were generated in an acoustic tank with degassed water in controlled temperature environment (23 °C) (Figure 1). A confocal two-element transducer with a focus length of 7 cm, a lateral resolution of 0.7 mm, and an axial focus size of 1 cm generated two ultrasound beams. The ceramics were driven by function generators (model 33220A, Agilent Technologies, Palo Alto, CA, USA) with slightly different frequency signals f ± Δf/2, where f is the central frequency of the ceramic equal to 3.2 MHz and Δf the frequency difference equal to 30 kHz. The signals were amplified by a homemade power amplifier before going to the ceramics. To detect the low frequency response, we used a wideband-preamplified hydrophone (frequency bandwidth: 0.03–70 kHz, sensitivity: −157 dB/1V/µPa, model ITC-6050C, International Transducer Corporation, Santa Barbara, CA, USA).

The face of the confocal transducer was positioned parallel to the surface of the cleft bone at a focal length of 7 cm. The hydrophone was fixed to the side of the transducer, out of the path of the ultrasonic beam (Figure 2). A picture showing the placement of the bone sample relatively to the confocal transducer can be seen in Figure 3.

To generate the images, the transducer focus was traced to the bone, according to the pattern shown in Figure 4, with a resolution of 0.25 mm, covering an area of 50 mm × 50 mm. Using the same experimental set-up five images of an intact femoral bone were generated for distances between the confocal transducer and bone: 6, 7, 8, 9 and 10 cm.
Results
This pilot study showed that VA images might be suitable to evaluate bone tissue at least in the experimental setup. The acquired images correspond to scanned regions of 2.5 cm × 2.5 cm, with acquisition time for high-resolution VA images (0.25 mm) of approximately 35 minutes.
In Figure 5 it is possible to observe the fracture of the distal femur both in the original bone (A) and generated image (B,C). In the intermediate region of the fracture, there is a blurring of the image related to the focus positioning.
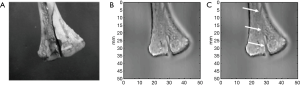
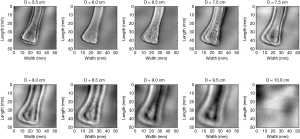
Figure 6 shows images of intact bone using different distances between the confocal transducer and the bone, which entails different positions of the transducer focus on the bone. At 10 cm bone image was completely unfocused.
Discussion
According to this preliminary study, VA may be suitable for the diagnosis of bone fractures in vitro. The acquisition time for high-resolution VA images (0.25 mm) is approximately 35 minutes using the current acquisition system. The acquisition time can be significantly reduced with the implementation of VA in clinical systems (19) using multi-element transducers (20). Future studies are encouraged to test if the technique may be suitable for the diagnosis in vivo of small bone fractures.
The contrast in the VA image when the bone was placed at different distances to the transducer, suggest the potential of VA image to evaluate the mineralization of internal structure of the bone. The dark contour around the bone is due to the scattering of the beam at the edge of the bone.
Unlike X-ray techniques, in VA there is no overlay of images when using high fundamental frequencies (greater than 1 MHz). The use of fundamental frequencies f1 and f2 of the order of 3 MHz allowed selective image generation focusing on the superficial region of the bone. This is because frequencies above 1 MHz have little penetration of the beam in bone, preventing the response of more internal regions. Figure 5 shows the possibility of selecting the region responsible to generate the image details by changing the transducer focus position.
Non-linear acoustic measurements were used to assess micro cracks in trabecular bone (21). More recently nonlinear resonant ultrasound spectroscopy technique has been used to monitor fatigue cortical bone micro damage (22). Ultrasonometry may be used to identify bone fractures in experimental and in vivo studies and to monitor fracture healing (23-27). To our knowledge, we found no previous papers showing VA images of bone fractures.
Our study has limitations that deserve mention. First, we evaluated only one case of femoral fracture. The evaluation of different types of fracture morphology should be performed to confirm the potential use of the technique. Second, the time for image acquisition need to be decreased to allow future clinical application. The current set-up is not suitable for clinical use, though the preliminary results should encourage further research and development.
VA use would prevent patient exposure to ionizing radiation inherent to X-ray techniques used for bone fracture diagnosis in the clinical routine. The high contrast generated by differences in the trabecular structure in the bone potentiates the use of this technique to evaluate the trabecular variation in osteoporotic bones.
Conclusions
The results reported here show that VA is capable of detecting bone fractures in vitro in high resolution images. Furthermore, the VA signal is associated with mechanical properties of the tissue. Thus, the acoustic parameters can be tuned to bring structural information of bones and help detecting conditions such as osteoporosis, as previously reported by other groups. VA is a promising technique and its implementation in a clinical system may bring a relatively low-cost and ionizing radiation-free alternative to X-ray to the clinical routine.
Acknowledgements
We thank the technical support of Agnelo dos Santos Bastos Neto, Carlos Renato da Silva, José Luiz Aziani and the company Figlabs Pesquisa e Desenvolvimento Ltda.
Funding: The authors acknowledge the funding support from FAPESP 11/10809-6, CNPq 304861/2013-8, CNPq 448806/2014-2 and Finep 2210/2008.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The approval of the Institutional Review Board was not necessary because no animal was sacrificed or submitted to experimental investigation.
References
- Cobbold RS. Foundations of Biomedical Ultrasound. Oxford University Press, 2006.
- Oldenburg AL, Boppart SA. Resonant acoustic spectroscopy of soft tissues using embedded magnetomotive nanotransducers and optical coherence tomography. Phys Med Biol 2010;55:1189. [Crossref] [PubMed]
- Kinney JH, Gladden JR, Marshall GW, Maynard JD. Resonant ultrasound spectroscopy measurements of the elastic constants of human dentin. J Biomech 2004;37:437-41. [Crossref] [PubMed]
- Dean LW. Interactions between sound waves. J Acoust Soc Am 1962;34:1039-44. [Crossref]
- Fatemi M, Greenleaf JF. Ultrasound-stimulated vibro-acoustic spectrography. Science 1998;280:82-5. [Crossref] [PubMed]
- Chen S, Fatemi M, Kinnick R, Greenleaf JF. Comparison of stress field forming methods for vibro-acoustography. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:313-21. [Crossref] [PubMed]
- Silva GT, Bandeira A. Difference-frequency generation in nonlinear scattering of acoustic waves by a rigid sphere. Ultrasonics 2013;53:470-8. [Crossref] [PubMed]
- Fatemi M, Greenleaf JF. Vibro-acoustography: An imaging modality based on ultrasound-stimulated acoustic emission. Proc Natl Acad Sci U S A 1999;96:6603-8. [Crossref] [PubMed]
- Silva GT, Chen S, Greenleaf JF, Fatemi M. Dynamic ultrasound radiation force in fluids. Phys Rev E Stat Nonlin Soft Matter Phys 2005;71:056617. [Crossref] [PubMed]
- Silva GT. Dynamic radiation force of acoustic waves on solid elastic spheres. Phys Rev E Stat Nonlin Soft Matter Phys 2006;74:026609. [Crossref] [PubMed]
- Silva GT, Urban MW, Fatemi M. Multifrequency radiation force of acoustic waves in fluids. Physica D: Nonlinear Phenomena 2007;232:48-53. [Crossref]
- Silva GT. Dynamic radiation force of acoustic waves on absorbing spheres. Braz J Phys 2010;40:184-7. [Crossref]
- Baggio AL, Kamimura H, Lopes JH, Silva GT. Parametric array signal in confocal vibro-acoustography. Applied Acoustics 2017;126:143-8. [Crossref]
- Fatemi M, Wold LE, Alizad A, Greenleaf JF. Vibro-acoustic tissue mammography. IEEE Trans Med Imaging 2002;21:1-8. [Crossref] [PubMed]
- Alizad A, Walch M, Greenleaf JF, Fatemi M. Vibrational characteristics of bone fracture and fracture repair: application to excised rat femur. J Biomech Eng 2006;128:300-8. [Crossref] [PubMed]
- Mitri FG, Davis BJ, Alizad A, Greenleaf JF, Wilson TM, Mynderse LA, Fatemi M. Prostate cryotherapy monitoring using vibro-acoustography: preliminary results of an ex vivo study and technical feasibility. IEEE Trans Biomed Eng 2008;55:2584-92. [Crossref] [PubMed]
- Callé S, Remenieras JP, Bou Matar O, Defontaine M, Patat F. Application of nonlinear phenomena induced by focused ultrasound to bone imaging. Ultrasound Med Biol 2003;29:465-72. [Crossref] [PubMed]
- Kamimura HA, Wang L, Carneiro AA, Kinnick RR, An KN, Fatemi M. Vibroacoustography for the assessment of total hip arthroplasty. Clinics (Sao Paulo) 2013;68:463-8. [Crossref] [PubMed]
- Urban MW, Chalek C, Kinnick RR, Kinter TM, Haider B, Greenleaf JF, Thomenius KE, Fatemi M. Implementation of vibro-acoustography on a clinical ultrasound system. IEEE Trans Ultrason Ferroelectr Freq Control 2011;58:1169-81. [Crossref] [PubMed]
- Kamimura HA, Urban MW, Carneiro AA, Fatemi M, Alizad A. Vibro-acoustography beam formation with reconfigurable arrays. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:1421-31. [Crossref] [PubMed]
- Renaud G, Callé S, Remenieras JP, Defontaine M. Nonlinear acoustic measurements to assess crack density in trabecular bone. Int J Non Linear Mech 2008;43:194-200. [Crossref]
- Haupert S, Guérard S, Peyrin F, Mitton D, Laugier P. Non Destructive Characterization of Cortical Bone Micro-Damage by Nonlinear Resonant Ultrasound Spectroscopy. PLOS one 2014;9:e83599. [Crossref] [PubMed]
- Protopappas VC, Fotiadis DI, Malizos KN. Guided ultrasound wave propagation in intact and healing long bones. Ultrasound Med Biol 2006;32:693-708. [Crossref] [PubMed]
- Barbieri G, Mazzer N, Ribeiro EA, Nogueira-Barbosa MH, Barbieri CH. A Comparative Analysis between Ultrasonometry and Computer-Aided Tomography to Evaluate Bone Healing. J Orthop Res 2012;30:1076-82. [Crossref] [PubMed]
- Bezuti MT, Mandarano LG, Barbieri G, Mazzer N, Barbieri CH. Ultrasonometry evaluation of axial compression osteosinthesis. An experimental study. Acta Ortop Bras 2013;21:46-51. [Crossref] [PubMed]
- Barbieri G, Barbieri CH. A comparative in vivo ultrasonometric evaluation of normal and delayed fracture healing in sheep tibiae. Clinics 2014;69:634-40. [Crossref] [PubMed]
- Mandarano-Filho LG, Bezuti MT, Barbieri CH. In vivo standardization of bone ultrasonometry of the clavicle. Clinics 2016;71:140-4. [Crossref] [PubMed]