Usefulness of enhancement-perfusion mismatch in differentiation of CNS lymphomas from other enhancing malignant tumors of the brain
Introduction
CNS lymphoma, glioblastoma multiforme (GBM), grade III enhancing tumors and metastasis are the common enhancing malignant brain tumors. Differentiating the above mentioned malignant tumors can be a challenge on routine MR imaging because of overlapping imaging findings (1). It is critical to distinguish CNS lymphomas from other enhancing malignant lesions preoperatively due to distinct prognostic implications and differing surgical planning and treatment options. Moreover, appropriate preoperative diagnosis of CNS lymphoma helps in averting needless surgical removal due to lack of survival benefits and increase in postoperative morbidities (2).
Indexed literature has proved promising role of utilizing advanced imaging techniques to help differentiation between the previously mentioned tumors. Dynamic susceptibility weighted magnetic resonance (DSC-MR) perfusion imaging is a fast and powerful functional MR technique, whose application is gaining ground in assessing intracranial tumors likely due to increasing availability of better hardware and software (3). Data provided by DSC perfusion MR imaging include physiological aspects of angiogenesis, vascular density and vascular endothelial proliferation (4,5). DSC-MR perfusion imaging is useful in characterization of tumor aggressiveness by evaluating tumor microcirculation and hemodynamics based on tumor neovascularization and endothelial hyperplasia (6). Relative cerebral blood volume (rCBV) is a most commonly used parameter in DSC-MR perfusion imaging. Several studies have shown, increase in the micro-vascularity and neo-vascularity of malignant intracranial masses correlate with high rCBV. GBM and high-grade gliomas have high rCBV due to rigorous neo-vascularity (6-8). Lymphomas were found to be associated with lower rCBV values (5,9,10). The rCBV values of lymphoma are noted to be much less compared to high-grade gliomas (11,12). Few earlier studies highlighted salient features of enhancing malignant lesions based on rCBV measurements on DSC perfusion and with diffusion (DWI-ADC), but were not always confirmatory (9,13). In previous studies, rCBV was chosen in the regions of interest with the maximum microvascular and neo-vascular density, which should represent the max rCBV. Several other techniques such as time-dependent leakage [permeability transfer constant (Ktrans)] reflecting leakiness on a T1W dynamic contrast-enhanced (DCE) perfusion, and fractional blood plasma volume per unit volume of tissue (Vp) reflecting perfusion have been used to measure perfusion-related parameters. DCE perfusion was also found effective in differentiating primary central nervous system lymphomas (PCNSL) from GBM (14,15). More recent studies have suggested the moderate effectiveness of DCE perfusion imaging for differentiation of GBM and PCNL (16).
However, the purpose of current study is to further explore the imaging role of DSC-MR perfusion. We hypothesized that enhancement-perfusion (E-P) mismatch on DSC perfusion imaging as measured by low mean rCBV in an enhancing portion of the tumor should allow differentiation of lymphomas from other enhancing malignant lesions.
Methods
Patient selection
This retrospective study group included 15 patients (13 biopsy proven primary and 2 secondary) and CNS lymphomas (10 males and 5 females), from July 2011 to December 2014. Inclusion criteria included the patients with parenchymal CNS lymphoma for which optimum pretreated DSC perfusion imaging were available. As a control group, histopathology proven matched 18 GBM (group II), 13 solitary intracranial metastases (group III), and 10 anaplastic enhancing gliomas (group IV) patients with optimum pretreatment DSC perfusion images were included from the same time period. Metastases were from lung (n=8), esophagus (n=2), breast (n=2) and melanoma (n=1) primaries. Inclusion criteria included only the patients with enhancing lesions on imaging and without prior treatment.
Imaging protocol
Various MR imaging sequences were obtained on a 1.5/3 T MR scanner (Signa LX Scanner, and DiscoveryTM 750; GE Healthcare, Milwaukee, Wisconsin, USA) with the help of standard 8-channel head coil. Conventional sequences included axial T1 FSE, T2FLAIR, T2 FSE, and gradient recalled echo (GRE). Post-contrast T1-weighted images in 3 planes were obtained following acquisition of DSC imaging. DSC images were acquired with T2* (gradient-echo echo planar) sequence using parameters which include: matrix size =128×96, section thickness =6 mm without gap, NEX 1, TR/TE =1,500/50 ms and flip angle =80°. Before starting the contrast agent injection, initial first 10 acquisitions in a total of 60 image volumes were selected to establish a pre-contrast baseline. After acquisition of the first 10 image volumes, a total of 0.15 mmol/kg of body weight gadopentetate dimeglumine was injected at a rate of 5 mL/s followed by a 20 mL bolus of saline injection at the same rate of 5 mL/s through an 18- or 20-G intravenous catheter. A total of 12 contiguous axial sections were chosen for the analysis on the basis of lesion extent determined by the pre-contrast T2 FLAIR images.
Post processing and perfusion measurements
rCBV measurement
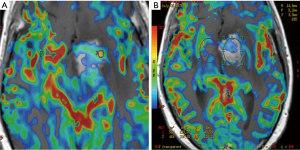
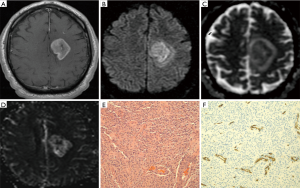
Post-processing of images was conducted on a different work station by author (R Mangla), who was blinded from the histologic findings at the time of analysis. Reference was made with conventional imaging during calculation of rCBV. Region of interests (ROIs) were drawn around the most enhancing part of tumor on contrast-enhanced T1WI axial images and images were transferred onto co-registered DSC perfusion maps multiple ROIs of 20–50 mm2 were drawn over 4 or 5 hot perfused spots, and the highest value i.e., max rCBV was picked (Figure 1A). This method has been shown to have a better inter- and intra-observer agreement (4). Raw data of perfusion images as well as T1- and T2-weighted images were used to ensure that regions of interest did not include apparent blood vessels or any hemorrhage. For normalization, another ROI with approximate size of 20–50 mm2 was drawn in the contralateral normal-appearing white matter as a standard internal reference. In this way, CBV measurements become a relative measure and are called rCBV. After that, rCBV ratio was obtained by dividing the rCBV values of lesion from the contralateral normal-appearing white matter. Similarly, mean rCBV was measured in the section with the largest enhancing dimensions and a ROI was placed to cover almost the entire portion of the tumor (Figure 1B). More the E-P mismatch, lower the mean rCBV.
Statistical analysis
Various perfusion parameters were analyzed for diagnostic accuracy by using SAS, Version 9.4 (SAS Institute, Cary, North Carolina, USA) and the IBM SPSS statistics, Version 22.0 (IBM, Chicago, Illinois, USA) software. Significance of the results between the groups was tested by Mann-Whitney test. Receiver operating characteristic (ROC) was analyzed for various perfusion parameters with statistically significant differences in regard to their ability to differentiate lymphoma from GBM, lymphoma from metastases and lymphoma from anaplastic enhancing tumors. The area under the curve (AUC) was computed to determine most predictive continuous variables among max rCBV and mean rCBV for diagnosing lymphomas.
Results
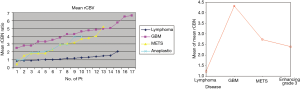
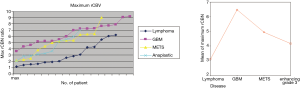
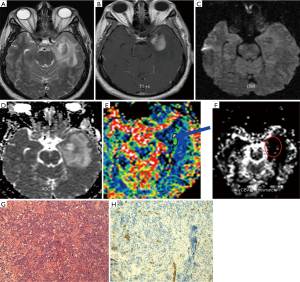
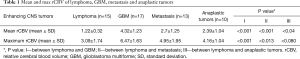
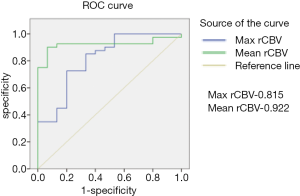
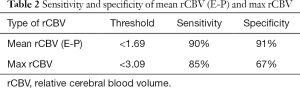
The mean rCBV and max rCBV of lymphomas, GBMs, metastases and anaplastic enhancing malignant tumors are summarized in Figures 2,3 respectively. Representative PCNSL and GBM cases are shown in Figures 4,5. The enhancing components of CNS lymphomas were found to have significantly lower mean rCBV (E-P mismatch) compared to enhancing component of GBM (1.2 versus 4.3; P<0.001) (Table 1; Figure 2), metastasis (1.2 versus 2.7; P<.001) (Table 1; Figure 3), and anaplastic enhancing tumors (1.2 versus 2.4; P<0.001) (Table 1; Figure 2). Maximum rCBV of enhancing component of lymphomas were also low but only significantly lower than GBM (3.1 versus 6.5; P<0.001) (Table 1, Figure 3) and metastasis (3.1 versus 4.9; P<0.013) (Table 1; Figure 3) but not with anaplastic enhancing tumors (3.9 versus 4.2; P<0.08) (Table 1; Figure 3). On the basis of ROC analysis, mean rCBV provided the best threshold (AUC =0.92) and had better accuracy in differentiating malignant lesions (Figure 6). Table 2 shows the sensitivity and specificity of mean rCBV (E-P) in differentiating these lesions. In our study, low mean rCBV (E-P mismatch) had better sensitivity (90%) and specificity (91%) with a cut-off <1.7 in differentiating lymphoma from other malignant intracranial lesions.
Full table
Full table
Discussion
Diagnosis of lymphoma pre-operatively is crucial due to distinct prognostic implications and differing surgical planning and treatment options, as well as to guide for biopsy (17,18). Steroid administration prior to histological diagnosis of brain tumors, can result in decreased diagnostic yield for resection or stereotactic biopsies, therefore making it imperative to recognize lymphoma by imaging (19). Despite similar MR imaging findings, significant structural differences in the tumor capillaries have been documented on reported histological studies (20). Differences in angio-architecture cause variability in DSC-MR perfusion parameters such as rCBV, which reflects the micro-vessel density (MVD), and PSR (perfusion signal intensity recovery which is measured at the end of contrast agent first pass, compared to baseline) as well as in DCE-MRI parameter such as Ktrans, which is the coefficient of volume transfer denoting vascular permeability and can help in differentiating these tumors.
DSC-MR perfusion imaging is based on a first-pass bolus imaging technique, which relies on the susceptibility induced signal loss on T2* weighted sequences resulting from a bolus of gadolinium-based contrast passing through a capillary bed. This is generally used to estimate rCBV in brain tumor studies, which can reflect tumor vascular morphometry (21). On DSC-MR perfusion imaging, gadolinium leaks into the interstitial spaces of the enhancing tumors and therefore, alters the microenvironment and subsequently signal-intensity recovery curve shape. T1 effects result in higher signal-intensity recovery, while T2* effects result in lower signal-intensity recovery (22). Due to complex interplay of multiple mechanisms at the time of passage of gadolinium as well as after passage of gadolinium during PSR and Ktrans measurements (23), there was a need of simpler parameter for assessing permeability.
It has been documented that area with maximal-rCBV does not correspond to the area of highest permeability and only modest correlation has been found between max rCBV and Ktrans (4). Microvascular area (MVA), that encompasses both the number and the caliber of the micro vessels, can provide a better estimation of the whole vascular surface area and represents a better and definite measure of the degree of angiogenesis (24). Perfusion has been shown to have high correlation with the MVA and poor correlation with permeability (25). However, permeability has shown moderate correlation with maximal enhancement (25). Based on the above facts, it was presumed that another simple parameter of DCS perfusion imaging should be evaluated which accounts for both perfusion and permeability. We hypothesized that E-P mismatch in an enhancing portion of the tumor, which is represented by low mean rCBV on DCS perfusion imaging, should allow differentiation of enhancing malignant lesions. Indeed in our study, we found that CNS lymphomas can be differentiated from other enhancing malignant lesions based on E-P mismatch.
Differentiating lymphoma from GBM and anaplastic enhancing tumors
In our study, lymphomas were found to have lower max rCBV values than high-grade gliomas, similar to previous studies (9-12). However, max rCBV values of anaplastic enhancing tumors were not significantly different from max rCBV of lymphomas (4.16 vs. 3.09, P<0.08). Lymphomas have unique angiocentric growth patterns with invasion of lumen vessel as well as endothelial cells with paucity of neo-angiogenesis and small extravascular extracellular space (26), resulting in less MVA and hence low perfusion. Also, frank endothelial discontinuities (due to thin and fenestrated endothelial cells) lead to high permeability resulting in frequently noted intense gadolinium enhancement in CNS lymphomas (27). Thus, lymphomas should have high E-P mismatch, as evident by statistically significant lower mean rCBV compared to GBMs and anaplastic tumors in our studies. GBMs have fragile neo-capillaries, disrupted blood brain barrier and vascular hyperplasia however, the MVA has been found to be less than anaplastic glioma due to lack of endothelial proliferation (22,23,28). These peculiar angio-architectures result in high perfusion and relatively low enhancement in anaplastic tumors compared to GBMs, as evident by more E-P mismatch or relatively lower mean rCBV values in anaplastic tumors compared to GBMs but significantly higher mean rCBV values compared to lymphomas in our study. In summary, lymphomas were difficult to differentiate from anaplastic gliomas on the basis of max rCBV; however, mean rCBV was useful.
Differentiating lymphoma from metastasis
Metastatic lesions show fenestrations in their tumor capillaries corresponding to primary systemic cancers with lack of blood-brain barrier, leading to high permeability (29). It has been found that max rCBV of an enhancing mass stands as a good criterion to differentiate metastases from lymphoma and GBM (30). In our study, there was significant difference in both max rCBV (3.09 vs. 4.95, P<0.013) and mean rCBV (1.22 vs. 2.7; P<0.001) values of lymphomas and metastases. However, mean rCBV proved to be more robust than max rCBV in differentiation.
Limitations
This retrospective study has several limitations. Many of our patients were treated with steroids based on clinical indications, which may affect the perfusion parameters. Technical limitations such as susceptibility artifacts related to hemorrhage, can lead to miscalculations in rCBV. Also, MR acquisition protocol can affect rCBV. A few lesions were assumed to be metastases based on primary neoplasm at other sites, without histopathology correlation. We want to mention that the reported results are purely observational using the proposed protocol using image processing methods in a small number of patients with lymphomas, GBMs, metastases, anaplastic gliomas.
Conclusions
DSC-perfusion MR imaging can be useful in differentiation of lymphoma from other enhancing malignant brain tumors. Pretreatment mean and max rCBV were found to be low in lymphomas when compared to other enhancing malignant brain tumors in the present study. ROC showed that mean rCBV (E-P mismatch) had better sensitivity (90%) and specificity (91%) with a cut-off <1.7 in differentiating lymphoma from other malignant brain tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by University of Rochester Medical Center Ethical Committee (No. RSRB00023950) and written informed consent was obtained from all patients.
References
- Tang YZ, Booth TC, Bhogal P, Malhotra A, Wilhelm T. Imaging of primary central nervous system lymphoma. Clin Radiol 2011;66:768-77. [Crossref] [PubMed]
- Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 2000;92:261-6. [Crossref] [PubMed]
- Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 2004;9:528-37. [Crossref] [PubMed]
- Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 2004;25:746-55. [PubMed]
- Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M. Evaluation of different cerebral mass lesions by perfusion weighted MR imaging. J Magn Reson Imaging 2006;24:817-24. [Crossref] [PubMed]
- Folkerth RD. Descriptive analysis and quantification of angiogenesis in human brain tumors. J Neurooncol 2000;50:165-72. [Crossref] [PubMed]
- Folkerth RD. Histologic measures of angiogenesis in human primary brain tumors. Cancer Treat Res 2004;117:79-95. [Crossref] [PubMed]
- Sharma S, Sharma MC, Gupta DK, Sarkar C. Angiogenic patterns and their quantitation in high grade astrocytic tumors. J Neurooncol 2006;79:19-30. [Crossref] [PubMed]
- Weber MA, Zoubaa S, Schlieter M, Juttler E, Huttner HB, Geletneky K, Ittrich C, Lichy MP, Kroll A, Debus J, Giesel FL, Hartmann M, Essig M. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 2006;66:1899-906. [Crossref] [PubMed]
- Lee IH, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS. Analysis of perfusion weighted image of CNS lymphoma. Eur J Radiol 2010;76:48-51. [Crossref] [PubMed]
- Cho SK, Na DG, Ryoo JW, Roh HG, Moon CH, Byun HS, Kim JH. Perfusion MR imaging: clinical utility for the differential diagnosis of various brain tumors. Korean J Radiol 2002;3:171-9. [Crossref] [PubMed]
- Liao W, Liu Y, Wang X, Jiang X, Tang B, Fang J, Chen C, Hu Z. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol 2009;50:217-25. [Crossref] [PubMed]
- Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 2006;58:394-403. [Crossref] [PubMed]
- Kickingereder P, Sahm F, Wiestler B, Roethke M, Heiland S, Schlemmer HP, Wick W, von Deimling A, Bendszus M, Radbruch A. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol 2014;35:1503-8. [Crossref] [PubMed]
- Abe T, Mizobuchi Y, Nakajima K, Otomi Y, Irahara S, Obama Y, Majigsuren M, Khashbat D, Kageji T, Nagahiro S, Harada M. Diagnosis of brain tumors using dynamic contrast-enhanced perfusion imaging with a short acquisition time. Springerplus 2015;4:88. [Crossref] [PubMed]
- Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V, Omuro A, Arevalo-Perez J, Thomas AA, Huse J, Peck K, Holodny AI, Young RJ. Diagnostic Accuracy of T1-Weighted Dynamic Contrast-Enhanced-MRI and DWI-ADC for Differentiation of Glioblastoma and Primary CNS Lymphoma. AJNR Am J Neuroradiol 2017;38:485-91. [Crossref] [PubMed]
- Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol 2005;44:728-34. [Crossref] [PubMed]
- Küker W, Nagele T, Korfel A, Heckl S, Thiel E, Bamberg M, Weller M, Herrlinger U. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 2005;72:169-77. [Crossref] [PubMed]
- Porter AB, Giannini C, Kaufmann T, Lucchinetti CF, Wu W, Decker PA, Atkinson JL, O'Neill BP. Primary central nervous system lymphoma can be histologically diagnosed after previously corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol 2008;63:662-7. [Crossref] [PubMed]
- Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol 1997;32:253-65. [Crossref] [PubMed]
- Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 2012;14:942-54. [Crossref] [PubMed]
- Hakyemez B, Yildirim N, Erdoğan C, Kocaeli H, Korfali E, Parlak M. Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation? Neuroradiology 2006;48:695-702. [Crossref] [PubMed]
- Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 2006;27:859-67. [PubMed]
- Korkolopoulou P, Patsouris E, Kavantzas N, Konstantinidou AE, Christodoulou P, Thomas-Tsagli E, Pananikolaou A, Eftychiadis C, Pavlopoulos PM, Angelidakis D, Rologis D, Davaris P. Prognostic implications of microvessel morphometry in diffuse astrocytic neoplasms. Neuropathol Appl Neurobiol 2002;28:57-66. [Crossref] [PubMed]
- Kiessling F, Krix M, Heilmann M, Vosseler S, Lichy M, Fink C, Farhan N, Kleinschmidt K, Schad L, Fusenig NE, Delorme S. Comparing dynamic parameters of tumor vascularization in nude mice revealed by magnetic resonance imaging and contrast-enhanced intermittent power Doppler sonography. Invest Radiol 2003;38:516-24. [Crossref] [PubMed]
- Kleihues P, Burger PC, Scheithauer BW. Histological Typing of Tumours of the Central Nervous System. Berlin, Germany: Springer-Verlag, 1999.
- Molnár PP, O'Neill BP, Scheithauer BW, Groothuis DR. The blood-brain barrier in primary CNS lymphomas: ultrastructural evidence of endothelial cell death. Neuro Oncol 1999;1:89-100. [Crossref] [PubMed]
- Birner P, Piribauer M, Fischer I, Gatterbauer B, Marosi C, Ambros PF, Ambros IM, Bredel M, Oberhuber G, Rössler K, Budka H, Harris AL, Hainfellner JA. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol 2003;13:133-43. [Crossref] [PubMed]
- Long DM. Capillary ultrastructure in human metastatic brain tumors. J Neurosurg 1979;51:53-8. [Crossref] [PubMed]
- Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002;222:715-21. [Crossref] [PubMed]


