Diagnostic imaging features of necrotizing enterocolitis: a narrative review
Introduction
Newborn necrotizing enterocolitis (NEC) is a term used to describe a common and devastating gastrointestinal neonatal pathology (1-3). NEC is an inflammatory process, characterized by intestinal necrosis of variable extension, leading to perforation, generalized peritonitis and death (1,4). It is the most common gastrointestinal emergency in the newborn (1-3,5,6), more frequent in the preterm infant, especially those of very low weight [newborns with birth weight <1,500 g or very low birth weight (VLBW)], although it affects term infants also (5,7-10). The incidence of NEC in VLBW infants, stable over the years, is between 5–7% (11,12); a slight increase over the period 2000–2009 was reported in the intensive care unit neonatal (NICU) of the Vermont Oxford Network (VON) (12). Recently in UK an incidence of around 3% in infants <32 weeks’ gestational age, between 2012 and 2013, was reported (13). Mortality from NEC varies depending from the degree of bowel involvement and comorbidities, up to 50% in those forms which require surgical treatment (1-3,6,14). The age of onset of NEC is inversely proportional to gestational age (15); those born at term develop NEC much earlier than pre-term infants, with the average age of the onset by the 1st week of life, particularly within the first 2–3 days (16).
Described for the first time in 1938 in a case of fetal peritonitis (17), the NEC is a disease linked to medical progress; its incidence increased with improvement of neonatal resuscitation techniques that enabled the survival of premature of VLBW. In addition, the dramatic prolongation of hospital stay for perinatal and neonatal care exposed the preterm at a higher risk of NEC (5).
According to the classical pathogenetic theory formulated in 1969 by Lloyd (18), the NEC in premature infant should be the final event of a “cascade” that, starting from a “stress induced” intestinal ischemia, leads to alterations of intestinal mucosa with translocation and bacterial colonization of the wall, perforation and death. A new pathogenetic hypothesis instead explains the NEC as a result of a series of imbalances caused by the immaturity of the gastrointestinal system, digestive system, circulatory regulation and of the immune system of the newborn. Intestinal immaturity leads to a compromised intestinal epithelial barrier, an underdeveloped immune defense, and altered vascular development and tone, so the newborn is more exposed to intestinal inflammation and sepsis (19,20).
Clinical presentation
Clinical presentation of NEC in newborn ranges from mild forms with moderate gastrointestinal tract disorder and that can heal spontaneously, to very serious forms with fulminant course characterized by perforation, peritonitis, sepsis, disseminated intravascular coagulation (DIC) and shock (21). First signs usually appear after start eating with nutritional intolerance: distension/abdominal discomfort, delayed gastric emptying and gastric retention and vomiting, first inconstant then biliary (8,22,23). Diarrhea, ematic or not, may occur (1,21). Furthermore, in almost all newborns a reduced peripheral circulation was noticed (24). Blushing, swelling and resistance of the abdominal wall are commonly described in the advanced stages of the NEC (25).
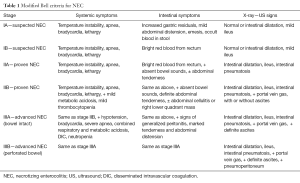
Nonspecific signs and symptoms such as worsening apnea, bradycardia, lethargy and instability of body temperature may be the first manifestations of the disease with insidious progression (26-28). General conditions become quickly compromised and, in the advanced stages, necrosis of the intestinal mucosa leads to perforation, peritonitis, sepsis with consequent persistent acidosis, clotting disorders, circulatory collapse and shock (29-31). Clinical suspicion is essential for an early recognition and an effective treatment to drastically reduce the morbidity and mortality of the disease. In 1978 Bell (26) proposed a clinical staging of the disease, modified in 1986 by Walsh and Kliegman (21), particularly useful in the evaluation of the patient and his treatment (Table 1).
Full table
According this classification, stage 1 (suspected NEC) is associated with clinical and radiological signs not specific such as body temperature instability, apnea, bradycardia, lethargy, moderate abdominal distension, fecal occult blood, dilated bowel loops with moderate ileus. Stage 2 (proven NEC) is characterized by rectal bleeding, moderate metabolic acidosis and thrombocytopenia together with radiological signs of ascites (due to the peritoneal inflammatory reaction to bacterial invasion) and intestinal pneumatosis. Typically, a linear strip of gas in the wall thickness of a limited portion of the intestine is observed. Rarely, it can involve both the small and large intestine. Moreover, often the bubble pattern is very similar to those infants with meconium retention; so, this sign is less specific than the former. In this stage it is also possible to observe an abdominal mass in the right lower quadrant. Finally, stage 3 (advanced NEC) is characterized by hypotension, DIC and often signs of generalized peritonitis. Respiratory acidosis associated with radiological signs of intestinal perforation can be present.
Imaging
Imaging modality in the diagnosis of NEC is historically represented by the plain-film abdominal radiographs (32) which can be performed every 6 hours because of the rapid evolution that may occur in the patient’s clinical condition. Ultrasound (US), in recent years, is playing an increasingly important role in the evaluation of early stages of the disease. The basic premise for understanding the role of imaging is to know the pathophysiological “cascade” that leads to NEC. Reduction of bowel wall perfusion leads to local ischemia with damage of the mucosal barrier resulting in penetration of air (pneumatosis) and microorganisms.
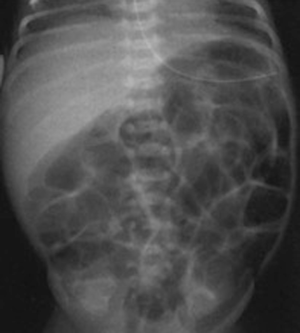
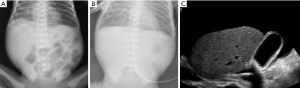
Radiographic findings have been well described in the literature (33,34), ranging from completely unspecific signs, such as a widespread bowel distension (Figure 1), up to more useful signs as wall thickening, fixation of the loops or reduction of intestinal air (Figure 2). X-ray abdomen examination shows a specific pattern only when there is the mucosal damage with pneumatosis of the intestinal wall and pneumoperitoneum (Figure 3). This is an already advanced stage of disease that may precede perforation and pneumoperitoneum, a condition that requires urgent surgical treatment and often leads to a negative outcome (35,36).
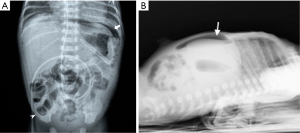
Recently a standardized evaluation of the plain-film X-ray of the abdomen using the Duke Abdominal Assessment Scale was proposed. It consists in a score based on radiological findings that increases with disease severity, ranging from 0 (normal gas pattern) to 10 (pneumoperitoneum) (37,38).
Despite the importance of the presence of pneumatosis there is a wide range of pathophysiological events related to bowel wall suffering that show a nonspecific radiographic pattern. So, the “goal” of the diagnostic path is to highlight precisely these early stages before bowel wall damage is determined. US is a fundamental tool to make an early diagnosis.
US has certain advantages over conventional X-ray examination (39-42), as it (I) provides images, in real time, of the abdominal structures, thus being able to assess the presence and validity of peristalsis of the bowel loops; (II) allows to detect the presence of even minimal amounts of fluid in the peritoneal cavity not detectable with standard X-ray; (III) allows to accurately detect the thickness of the intestinal wall and evaluate the presence, absence or reduction of the wall perfusion (40,41,43,44).
In the early stages of NEC, when X-ray can show only an aspecific loop distension, US shows direct and more specific signs as the following:
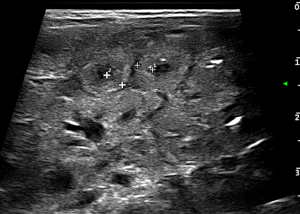
- Wall thickening: in general it is considered pathological a wall thickness greater than 2.6 mm (36,43) (Figure 4);
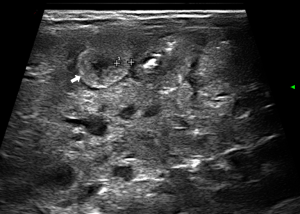
- Abnormal bowel wall echoic pattern: the reduction of normal wall layering results in a progressive increase in the parietal US echogenicity (43,45) (Figure 5);
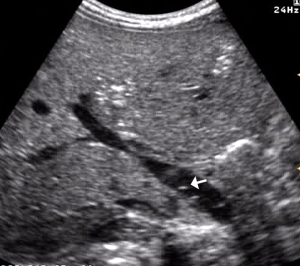
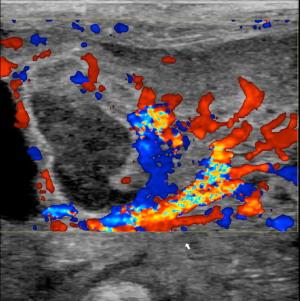
- Wall perfusion: in this stage there is an increase in vascularity of the wall and mesenterial perivisceral tissues (36,40,46) (Figure 6);
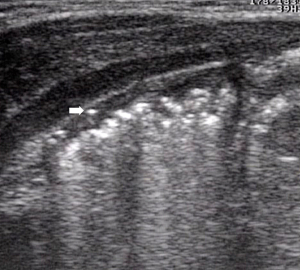
- Initial signs of intestinal pneumatosis: the presence of air microbubbles inside wall thickness appears as hyperechoic spots, usually without signs of posterior reverberation (46). This finding represents the sign of the air passage from the intestinal lumen, as a result of the damage of the mucosal barrier (Figure 7). It should be emphasized that, at this stage, the small amount of air detected by US is not appreciable at radiographic examination (36).
In the intermediate stage of NEC, when radiographic examination indicates signs of intestinal pneumatosis, US can show the following:
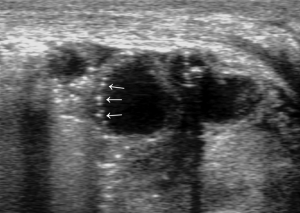
- Extensive penetration of air in the wall thickness area: presence of air appears as multiple hyperechoic spots limited to some continuous wall portions (Figure 8) or with a circumferential pattern (Figure 9) and affects one or more loops (42,43,47);
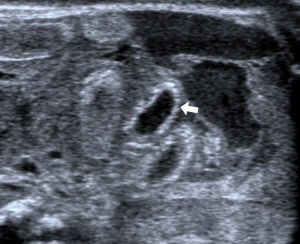
- Portal pneumatosis: air is present in the main portal vein (Figure 10) and in the peripheral intrahepatic portal branches. In the latter case the pneumatosis manifests as a series of hyperechoic spots, irregularly distributed in the liver parenchyma, often moving during the examination (Figure 11) (39,40,46,48);
- Extraintestinal gas: presence of small hyperechoic spots, expression of small air bubbles between the front surface of the liver and the abdominal wall or between the intestinal loops (initial sign of intestinal perforation). Also in this case the US is more sensitive than X-ray examination. The small amount of extraintestinal air, detectable with US, is not detectable in direct radiography (36,40).
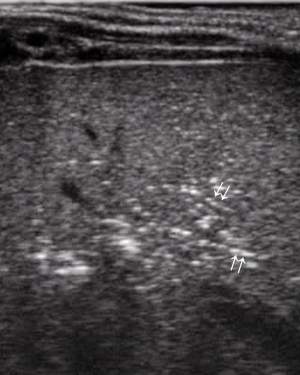
In advanced NEC, when radiographic examination demonstrates signs of pneumoperitoneum, US shows:
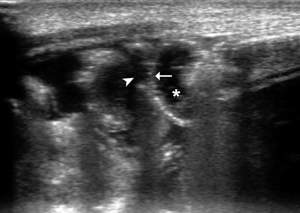
- Bowel wall ischemia: wall thinning (Figure 12). The real reduction in bowel wall thickness has to be distinguished from an apparent thinning due to “stretching” of the loop caused by fluid accumulation in the lumen in patients with and without NEC (40,42); significant reduction of the wall vascularization until its complete disappearance (40,43) (Figure 13);
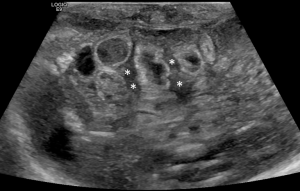
- Significant amount of free fluid in the abdomen: in the newborn, a small amount of free fluid between the loops can be present (36). The amount and echostructural aspect of the liquid can indicate disease. In fact, in the NEC, the presence of abundant amount of liquid that shows inhomogeneous echostructure with internal echoes and septa is suggestive of perforation (49) (Figure 14).
Conclusions
Since the NEC clinical signs, both early and late, are often non-specific (40), imaging plays an important role in the timing of diagnosis. While plain abdominal radiographs remain the main and most used modality in the evaluation and monitoring of the NEC, it is true that the most specific sign that defines the diagnosis is the presence of intramural gas (37,40,46,50,51). Unfortunately, this radiographic sign is present in the advanced stages of the disease when the damage of the wall has been consolidated leading perforation.
US offers several advantages that can potentially contribute to an earlier diagnosis or, at least, provide more timely information about the stage of the intestinal wall suffering. Timing of US follow-up is not yet standardized (40,42,45,46,51). However, greater results can occur in patients who present a worsening of the clinical symptoms with no obvious signs of intestinal pneumatosis and/or pneumoperitoneum on X-ray plain film. In these patients US, with the possibility to assess the vitality of the intestinal wall (with colour Doppler), the damage of the bowel wall (by identifying early signs of pneumatosis) and to recognize the presence of free fluid in the abdomen, may provide a prompt guide to clinical management, since its findings have a good relationship with patients outcomes (36,41,44,46,49,51,52).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271-83. [Crossref] [PubMed]
- Berman L, Moss RL. Necrotizing enterocolitis: An update. Semin Fetal Neonatal Med 2011;16:145-50. [Crossref] [PubMed]
- Dominguez KM, Moss RL. Necrotizing Enterocolitis. Clin Perinatol 2012;39:387-401. [Crossref] [PubMed]
- Jona JZ. Advances in neonatal surgery. Pediatr Clin North Am 1998;45:605-17. [Crossref] [PubMed]
- Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. Am J Dis Child 1981;135:603-7. [Crossref] [PubMed]
- Kim JH. Necrotizing enterocolitis: The road to zero. Semin Fetal Neonatal Med 2014;19:39-44. [Crossref] [PubMed]
- Wilson R, del Portillo M, Schmidt E, Suh MH, Yousefi S, Saunders LJ, Belghith A, Manalastas PI, Medeiros FA, Weinreb RN. Risk factors for necrotizing enterocolitis in infants weighing more than 2,000 grams at birth: a case-control study. Pediatrics 1983;71:19-22. [PubMed]
- Ng S. Necrotizing enterocolitis in the full-term neonate. J Paediatr Child Health 2001;37:1-4. [Crossref] [PubMed]
- Kosloske AM. Pathogenesis and prevention of necrotizing enterocolitis: a hypothesis based on personal observation and a review of the literature. Pediatrics 1984;74:1086-92. [PubMed]
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001;107:E1. [Crossref] [PubMed]
- Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, Buzas JS, Bertino E, Gagliardi L, Bellù R. Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000-2013. Pediatrics 2015;136:e84-92. [Crossref] [PubMed]
- Johnson TJ, Patel AL, Bigger HR, Engstrom JL, Meier PP. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015;107:271-6. [Crossref] [PubMed]
- Eaton S. Necrotizing enterocolitis symposium: Epidemiology and early diagnosis. J Pediatr Surg 2017;52:223-5. [Crossref] [PubMed]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. [Crossref] [PubMed]
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129:e298-304. [Crossref] [PubMed]
- Wright K, Miller HD. Evidence-based Findings of Necrotizing Enterocolitis. Newborn Infant Nurs Rev 2012;12:17-20. [Crossref]
- Simpson J. Notices of cases of peritonitis in the foetus in utero. Edin M S J 1938;50:390.
- Lloyd JR. The etiology of gastrointestinal perforations in the newborn. J Pediatr Surg 1969;4:77-84. [Crossref] [PubMed]
- Sharma R, Tepas JJ 3rd, Hudak ML, Wludyka PS, Mollitt DL, Garrison RD, Bradshaw JA, Sharma M. Portal venous gas and surgical outcome of neonatal necrotizing enterocolitis. J Pediatr Surg 2005;40:371-6. [Crossref] [PubMed]
- Tanner SM, Berryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA. Pathogenesis of Necrotizing Enterocolitis. Am J Pathol 2015;185:4-16. [Crossref] [PubMed]
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179. [Crossref] [PubMed]
- Cobb BA, Carlo WA, Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics 2004;113:50-3. [Crossref] [PubMed]
- Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. J Pediatr Gastroenterol Nutr 2009;48:437-42. [Crossref] [PubMed]
- Grant J, Denne SC. Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. J Pediatr 1991;118:928-32. [Crossref] [PubMed]
- Palazzi DL, Klein JO, Baker CJ. Bacterial Sepsis and Meningitis. In: Infectious Diseases of the Fetus and Newborn Infant 2006:47-95.
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1-7. [Crossref] [PubMed]
- Dimmitt RA, Moss RL. Clinical management of necrotizing enterocolitis. Neoreviews 2001;2:110-7. [Crossref]
- Huda S, Chaudhery S, Ibrahim H, Pramanik A. Neonatal necrotizing enterocolitis: Clinical challenges, pathophysiology and management. Pathophysiology 2014;21:3-12. [Crossref] [PubMed]
- Buras R, Guzzetta P, Avery G, Naulty C. Acidosis and hepatic portal venous gas: indications for surgery in necrotizing enterocolitis. Pediatrics 1986;78:273-7. [PubMed]
- Pickard SS, Feinstein JA, Popat R, Huang L, Dutta S. Short- and long-term outcomes of necrotizing enterocolitis in infants with congenital heart disease. Pediatrics 2009;123:e901-6. [Crossref] [PubMed]
- Sullivan BA, Fairchild KD. Predictive monitoring for sepsis and necrotizing enterocolitis toprevent shock. Semin Fetal Neonatal Med 2015;20:255-61. [Crossref] [PubMed]
- Berdon WE, Grossman H, Baker DH, Mizrahi A, Barlow O, Blanc WA. Necrotizing enteroclitis in the premature infant. Radiology 1964;83:879-87. [Crossref] [PubMed]
- Daneman A, Woodward S, de Silva M. The radiology of neonatal necrotizing enterocolitis (NEC) A review of 47 cases and the literature. Pediatr Radiol 1978;7:70-7. [Crossref] [PubMed]
- Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am 1999;37:1187-98. [Crossref] [PubMed]
- Kosloske AM, Musemeche CA, Ball WS Jr, Ablin DS, Bhattacharyya N. Necrotizing enterocolitis: Value of radiographic findings to predict outcome. AJR Am J Roentgenol 1988;151:771-4. [Crossref] [PubMed]
- McCarten KM. Ultrasound of the Gastrointestinal Tract in the Neonate and Young Infant with Particular Attention to Problems in the Neonatal Intensive Care Unit. Ultrasound Clin 2010;5:75-95. [Crossref]
- Coursey CA, Hollingsworth CL, Gaca AM, Maxfield C, Delong D, Bisset G 3rd. Radiologists’ agreement when using a 10-point scale to report abdominal radiographic findings of necrotizing enterocolitis in neonates and infants. AJR Am J Roentgenol 2008;191:190-7. [Crossref] [PubMed]
- Markiet K, Szymanska-Dubowik A, Janczewska I, Domazalska-Popadiuk I, Zawadzka-Kepczynska A, Bianek-Bodzak A. Agreement and reproducibility of radiological signs in NEC using The Duke Abdominal Assessment Scale (DAAS). Pediatr Surg Int 2017;33:335-40. [Crossref] [PubMed]
- Merritt CR, Goldsmith JP, Sharp MJ. Sonographic detection of portal venous gas in infants with necrotizing enterocolitis. AJR Am J Roentgenol 1984;143:1059-62. [Crossref] [PubMed]
- Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, Taylor G, Gerstle JT. Necrotizing enterocolitis: review of state-of-the-artimaging findings with pathologic correlation. Radiographics 2007;27:285-305. [Crossref] [PubMed]
- Shebrya NH, Amin SK, El-Shinnawy MA, Imam SS. Abdominal ultrasonography in preterm necrotizing enterocolitis. Is it superior to plain radiography? Egypt J Radiol Nucl Med 2012;43:457-63. [Crossref]
- Garbi-Goutel A, Brévaut-Malaty V, Panuel M, Michel F, Merrot T, Gire C. Prognostic value of abdominal sonography in necrotizing enterocolitis of premature infants born before 33 weeks gestational age. J Pediatr Surg 2014;49:508-13. [Crossref] [PubMed]
- Faingold R, Daneman A, Tomlinson G, Babyn PS, Manson DE, Mohanta A, Moore AM, Hellmann J, Smith C, Gerstle T, Kim JH. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology 2005;235:587-94. [Crossref] [PubMed]
- Bohnhorst B. Usefulness of abdominal ultrasound in diagnosing necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F445-50. [Crossref] [PubMed]
- Kamali K, Hosseini SR, Ardakani SM, Farnoodi MR. Complementory Value of Sonography in Early Evaluation of Necrotizing Enterocolitis. Pol J Radiol 2015;80:317-23. [Crossref] [PubMed]
- Muchantef K, Epelman M, Darge K, Kirpalani H, Laje P, Anupindi SA. Sonographic and radiographic imaging features of the neonate with necrotizing enterocolitis: Correlating findings with outcomes. Pediatr Radiol 2013;43:1444-52. [Crossref] [PubMed]
- Dilli D, Suna Oǧuz Ş, Erol R, Ozkan-Ulu H, Dumanlı H, Dilmen U. Does abdominal sonography provide additional information over abdominal plain radiography for diagnosis of necrotizing enterocolitis in neonates? Pediatr Surg Int 2011;27:321-7. [Crossref] [PubMed]
- Sharma R, Hudak ML. A Clinical Perspective of Necrotizing Enterocolitis: Past, Present, and Future. Clin Perinatol 2013;40:27-51. [Crossref] [PubMed]
- Palleri E, Kaiser S, Wester T, Arnell H, Bartocci M. Complex Fluid Collection on Abdominal Ultrasound Indicates Need for Surgery in Neonates with Necrotizing Enterocolitis. Eur J Pediatr Surg 2017;27:161-5. [PubMed]
- Coursey CA, Hollingsworth CL, Wriston C, Beam C, Rice H, Bisset G 3rd. Radiographic predictors of disease severity in neonates and infants with necrotizing enterocolitis. AJR Am J Roentgenol 2009;193:1408-13. [Crossref] [PubMed]
- He Y, Zhong Y, Yu J, Wang Z, Li L. Ultrasonography and radiography findings predicted the need for surgery in patients with necrotising enterocolitis without pneumoperitoneum. Acta Paediatr 2016;105:e151-5. [Crossref] [PubMed]
- Silva CT, Daneman A, Navarro OM, Moore AM, Moineddin R, Gerstle JT, Mittal A, Brindle M, Epelman M. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 2007;37:274-82. [Crossref] [PubMed]