Myocardial strain and symptom severity in severe aortic stenosis: insights from cardiovascular magnetic resonance
Introduction
Aortic stenosis (AS) is the most frequent native valvular heart disease and typically presents as calcified degeneration in adults of advanced age (affecting 2–7% of those aged over 65 years) (1). Current guidelines recommend aortic valve replacement (AVR) with the onset of symptoms (angina, exertional dyspnoea, syncope) or cardiac dysfunction [left ventricular ejection fraction (LVEF) <50%] as class I indications (2).
Calcific AS is associated with a long latent phase without symptoms, which varies widely in duration between individuals (3-6). In this asymptomatic group, 2-year event-free survival ranges from 20% to more than 50% (6). However, the onset of symptoms marks a bleak prognosis (7). Given the lack of a direct relation between hemodynamic severity and clinical outcome, guidelines indicate careful history taking at each visit as critical to proper patient management (7). However, symptom onset can be insidious and patients’ reports are often ambiguous as they subconsciously reduce their activities or incorrectly ascribe a decline to other causes (8).
Left ventricular hypertrophy occurs in AS reflecting compensation for increased mid-wall stress from chronic pressure overload (9). Eventually an afterload mismatch impairs LV performance despite preservation of ejection fraction (10). Indeed, impaired LVEF represents an end stage of AS with worse outcomes even in those who receive corrective treatment (11).
Assessment of myocardial deformation parameters provides superior quantification of systolic function, with increased sensitivity to subtle myocardial dysfunction than global LVEF (12) and holds prognostic value in patients with AS (13) even when asymptomatic (14). Cardiovascular magnetic resonance (CMR) tagging is accepted by many as the gold standard for measurement of circumferential and longitudinal myocardial strain (15,16) and has good demonstrated reproducibility (17).
The aim of this study was to objectively define LV systolic and diastolic function, using CMR strain quantification, across a spectrum of symptom profiles in patients with severe AS and to determine whether strain assessment could potentially contribute to the decision-making process, particularly in patients with preserved LVEF and equivocal symptoms.
Methods
Study population
Forty two patients with severe degenerative AS were prospectively recruited between July 2008 and December 2013 from the University Hospitals of Leeds and Leicester, UK. Based upon transthoracic echocardiography (TTE), severe AS was defined as an aortic valve area (AVA) of ≤1.0 cm2 or peak velocity >4 m/s. Clinical evaluation comprised the elucidation of symptoms (NYHA functional class, angina, syncope) and physical examination. Demographic data and risk factors for cardiovascular were also documented. Patients were then grouped into those reporting “no/mild” symptoms (NYHA class I, II) and those reporting “significant” symptoms (NYHA class III, IV, angina, syncope). We also enrolled 13 age-matched healthy volunteers who underwent an identical CMR protocol. Exclusion criteria included any patients with more than mild aortic regurgitation, any contraindication to CMR, and for the healthy volunteers, symptoms or history of heart disease, abnormal resting blood pressure or diabetes mellitus. The study was approved by a national ethics committee, complied with the Declaration of Helsinki and written informed consent was provided by participants.
CMR Protocol
For both patients and healthy volunteers, an identical scan protocol was performed at 1.5 T (Intera, Phillips Healthcare, Best, Netherlands or Avanto, Siemens Medical Systems, Erlangen, Germany). Multi-slice, multi-phase cine imaging was performed using a standard steady-state free procession pulse sequence in the short axis (8 mm thickness, 0 mm gap, 30 phases, in-plane spatial resolution 1.1 mm × 1.1 mm) ensuring full coverage of both left and right ventricles. Through-plane velocity encoded (VENC) phase contrast imaging was performed perpendicular to the aortic valve jet at the aortic sinotubular junction (typical VENC 250–500 cm/s, retrospective gating, slice thickness 6 mm, 40 phases, FOV 340 mm).
Complementary spatial modulation of magnetization (CSPAMM) imaging was carried out during a single breath hold at end expiration in the short axis orientation, at the apex, mid-, and basal LV [multishot echo planar imaging, flip angle sweep applied to the radiofrequency excitation pulses of subsequent cardiac phases, two orthogonal line tags acquired per slice, field of view: 300 mm, matrix 128 × 128, slice thickness 10 mm, tag separation 8 mm, typically 18 phases, repetition time/echo time (TR/TE) 30/6 ms, flip angle 25 degrees]. The “3 of 5 technique” was utilised to optimise consistency of slice positioning between visits and has been demonstrated to be highly reproducible (17).
Image analysis
All analysis was performed blinded, using commercially available software (QMass 7.5 and Qflow 7.2, Medis Medical Imaging Systems, Leiden, the Netherlands). Standard ventricular and valvular assessment was performed as previously described (18).
CSPAMM analysis was performed using a dedicated tagging analysis package (inTag© software, Creatis, Lyon, France). Epicardial and endocardial contours were drawn for each slice. A mid-myocardial contour was automatically calculated and contours were propagated through all cardiac phases. Strain was measured in the mid-myocardial layer which has previously been reported to be the most reproducible (17). Peak circumferential LV strain was measured for the three slices at apex, mid-ventricle, and base. Peak systolic and diastolic LV strain rates were measured from the mid-ventricular slice.
Feature tracking analysis was performed using commercially available software (cvi42, Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Endocardial and epicardial contours were drawn on a long-axis 4-chamber cine using a semi-automated process (Figure 1). Peak LV longitudinal strain and systolic and diastolic strain rates were measured.
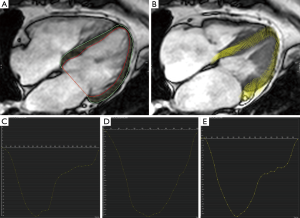
Statistical analysis
Continuous variables are presented as mean ± SD. The Shapiro-Wilk test was used to determine normality. Frequencies are reported as number (%). The Student t test and Wilcoxon signed rank test were used to compare continuous variables as appropriate. All statistical analyses were performed using the PASW software package (V.21.0 SPSS, IBM, Chicago, Illinois, USA) with a two-sided significance level of P<0.05 considered statistically significant.
Results
Study population
A total of 42 patients with severe AS were studied along with 13 healthy controls, matched for age, LVEF and body mass index (BMI) (Table 1). The patients with severe AS were further subdivided into two groups; those with “no/mild” symptoms (n=21), and those with “significant” symptoms (n=21). These two groups were comparable for age, surgical risk score, LV mass, measures of AS severity and history of ischaemic heart disease (Table 2). Across all three individual groups, average LVEF was above 50% (the defined cut-off for a class I indication for surgical valve replacement).
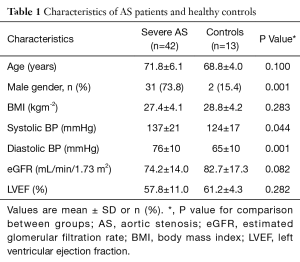
Full table
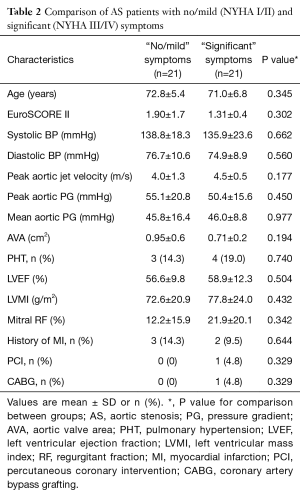
Full table
Comparison of healthy controls and severe AS
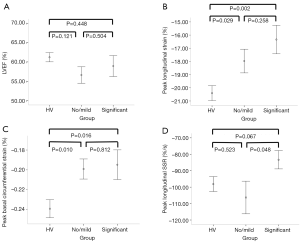
Compared to healthy controls matched for age and LVEF, patients with severe AS had significantly reduced (worse) peak longitudinal strain (PLS) (−17.1±4.50 vs. −20.4±2.04, P=0.001). Similarly they exhibited significantly reduced (worse) peak circumferential strain at basal (−19.7±5.8 vs. −23.9±3.2, P=0.015), mid (−21.3±5.3 vs. −25.9±3.8, P=0.005) and apical (−20.3±6.3 vs. −27.2±3.0, P=0.001) LV levels with significantly reduced peak circumferential systolic strain rate (−0.033±0.009 vs. −0.039±0.004, P=0.001). Even patients with “no/mild” symptoms were found to have significantly worse strain parameters when compared with healthy controls (Table 3). Peak longitudinal systolic strain rates were an exception to this, being comparable between healthy volunteers and those with severe AS, regardless of symptom profile.
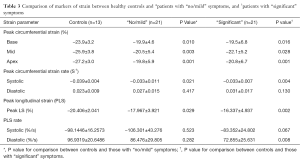
Full table
Comparison of severe AS patients reporting “no/mild” and “significant” symptoms
Compared to patients with “significant” symptoms, those reporting “no/mild” symptoms had statistically higher (better) peak longitudinal systolic strain rate (Figure 2), with comparable PLS and longitudinal diastolic strain rate. Peak circumferential strain at the basal, mid and apical LV levels and peak systolic and diastolic circumferential strain rates between the two symptom groups were also comparable (Table 4).

Full table
Discussion
This prospective two-centre study has comprehensively characterised LV mechanics using CMR in patients with severe AS and in healthy age-matched controls. Patients with severe AS exhibit diminished longitudinal and circumferential LV strain when compared to controls, despite preservation in LVEF. Importantly, we have demonstrated patients with severe AS and no or only minimal symptoms still exhibit LV systolic dysfunction that is comparable to those with significant symptoms who fulfil a class I indication for aortic valve surgery. CMR strain analysis may therefore have a future role in clarifying patient selection for AVR, particularly when symptoms are equivocal, given the ability to detect LV functional decline in the AS disease process more sensitively than global LV ejection fraction.
Valvular AS is a slowly progressive disease and symptom onset can be insidious. However, in patients with preserved systolic function, it is the significance of symptoms that clinically determines referral for surgery. In the Euro Heart Survey, 24% of patients with severe AS presented with congestive heart failure and the use of medical therapy was frequent. These observations suggest a sizeable group of patients with severe AS were managed at an advanced stage of their disease process (16). Mild symptoms are often denied or erroneously dismissed to advancing age or an intercurrent illness (8) which may potentially reduce their survival. The Euro Heart Survey reflects real world practise in which equivocal symptom reporting in the context of high risk aortic valve surgery is frequently managed conservatively.
The origin of exercise intolerance or symptoms in patients with severe AS is poorly understood (19) and there is considerable variation in the degree of stenosis associated with symptom onset. In our study, patients with no/mild and significant symptoms were comparable in age, LVEF, LV mass, mitral regurgitation, presence of pulmonary hypertension (PHT), history of coronary disease and aortic valve haemodynamics. It is notable LVEF is normal in most patients with severe AS, even when symptoms develop and that valve area and transvalvular gradients do not predict clinical outcomes following AVR (20). It can be challenging to clinicians to decipher a genuine change in symptoms attributable to AS. Similar to previous studies (19,21), our work highlights the need for novel objective measures of early LV decline that could guide a clinician to offering timely intervention.
This is the first study to use CMR strain analysis to attempt to differentiate patients with severe AS based on their symptomatic state. Our data suggests that those with significant symptoms (and thus clear surgical indication) have statistically worse peak longitudinal systolic strain rates than those with no/mild symptoms (in whom decision making can be challenging), despite other comparable measures of LV function by strain. Strain rate represents the rate of myocardial deformation and is an energy-requiring process (22). Compared with strain, it is less dependent upon pre-load, afterload and heart rate; and is representative of regional contractile function (23). Nonetheless, caution is required in interpreting this finding given the sample sizes, and larger study is required to clarify this reported depreciation of strain rate and its potential impact on clinical surveillance.
Our study using CMR strain analysis compares with other studies using echocardiography to evaluate LV function in patients with severe AS. Longitudinal strain appears invariably depressed with a number of reports indicating reduced circumferential strain also (9,21,24). Our patients with severe AS all had reduced circumferential strain when compared to age matched healthy controls with comparable ejection fraction. Previous CMR work has indicated pronounced myocardial steatosis is present in severe AS, and is independently associated with the degree of circumferential strain impairment, regardless of symptom state (25). Our work suggests lower longitudinal and circumferential strain is inherent to patients with severe AS irrespective of symptom status. As such, an incidental depressed strain measurement may not reliably position a patient in the disease timeline. Clinically, surveillance imaging will help objectively assess decline, and in particular deterioration of longitudinal systolic strain rate, may suggest loss of compensation and trigger surgical referral despite a normal LVEF.
Our study using CMR confirms severe valvular AS confers a chronic pressure overload that is detrimental to myocardial fibre function. This subtle detection of both systolic and diastolic LV dysfunction even in those with no or mild symptoms may explain previous findings that early elective AVR in asymptomatic patients affords beneficial early and late outcomes (26,27). It is noteworthy that delayed surgical treatment is the principal determinant of reduced late survival (27). Our observation of comparable impairment between those with significant symptoms, in whom AVR is clearly indicated, and those with no/mild symptoms suggests referral for surgery based on assessment of LV mechanics (and thus earlier than current guidelines would recommend) could avert a delay of prognostic importance. Indeed, our observation is noteworthy in light of recent data. In a sizeable multicentre registry comprising 3,815 patients, conservative management of asymptomatic severe AS patients was associated with a dismally higher incidence of mortality and hospital admission at 5 years when compared with an initial AVR approach (28). It is entirely possible the poor outcomes in these reportedly “asymptomatic” patients related to advanced subclinical LV dysfunction comparable to those with symptoms who would ordinarily have been intervened upon earlier.
A number of studies indicate the clinical importance of longitudinal strain measurement in patients with severe AS. It has the potential to predict recovery of LV dysfunction following surgery (9), the requirement for AVR in the future (29) and also independently predict all-cause mortality (30). Recently, deteriorating LV global longitudinal strain has also demonstrated incremental prognostic value in addition to symptom profile and surgical risk scoring; being superior to measures of aortic pressure gradient (PG) and stroke volume (13). Our study reflects this observation; demonstrating both longitudinal and circumferential strains are sensitive in detecting pathology before they are evident on conventional history taking. Given our observation of similar longitudinal strain between those with no/mild symptoms and those with significant symptoms, our study indicates the need to measure longitudinal strain in patients with ill-defined symptoms to determine whether they potentially fall into the same prognostic group as those with a manifest requirement for AVR.
Limitations
This was an observational study and associations do not prove causality. Our study population was relatively small and included patients with coronary artery disease and hypertension, rather than being restricted to isolated AS. This is however generalizable to real world clinical practice and reduces the effect of selection bias. Given the potential for type I statistical errors, our findings should be validated in a larger patient population to confirm any future clinical utility. We have used feature tracking, rather than CSPAMM, for the analysis of longitudinal strain. Tissue tagging is hampered by a lower temporal resolution and tag fading during diastole and feature tracking to determine longitudinal strain has demonstrated good reproducibility in patients with severe AS (31). Thresholds for normal versus pathological strain and strain rates may vary with different analysis software and pulse sequence acquisitions; thus our absolute values may not be translatable to other acquisition/analysis platforms.
Conclusions
In conclusion, LV deformation assessment using CMR offers a potentially valuable non-invasive assessment of patients with severe AS, more sensitive than the elucidation of symptoms at detecting a decline in LV performance. Patients with no or only mild symptoms exhibit comparable reduction in circumferential and longitudinal fibre function to those with significant symptoms in whom AVR is clearly indicated. Thus, CMR characterisation of LV strain may guide optimal decision making when caring for asymptomatic patients or those with borderline symptoms. Future larger prospective studies are needed to validate this interesting observation.
Acknowledgements
The authors thank Mrs Fiona Richards and Ms Petra Bijsterveld for their immense help in patient recruitment.
Funding: This study was part-funded by the British Heart Foundation (PG/11/126/29321) and also the National Institute for Health Research (NIHR) Leeds Clinical Research Facility. GP McCann is supported by a NIHR career development fellowship.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Ethical Statement: The study was approved by a national ethics committee (REC 08/H1307/106), complied with the Declaration of Helsinki and written informed consent was provided by participants.
References
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M, ESC Committee for Practice Guidelines (CPG). Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262-70. [Crossref] [PubMed]
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7. [Crossref] [PubMed]
- Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290-5. [Crossref] [PubMed]
- Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation 2010;121:151-6. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Otto CM. Aortic stenosis--listen to the patient, look at the valve. N Engl J Med 2000;343:652-4. [Crossref] [PubMed]
- Delgado V, Tops LF, van Bommel RJ, van der Kley F, Marsan NA, Klautz RJ, Versteegh MI, Holman ER, Schalij MJ, Bax JJ. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J 2009;30:3037-47. [Crossref] [PubMed]
- Ross J Jr. Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis 1976;18:255-64. [Crossref] [PubMed]
- Vaquette B, Corbineau H, Laurent M, Lelong B, Langanay T, de Place C, Froger-Bompas C, Leclercq C, Daubert C, Leguerrier A. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005;91:1324-9. [Crossref] [PubMed]
- Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011;8:494-501. [Crossref] [PubMed]
- Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging 2014;7:938-45. [Crossref] [PubMed]
- Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr 2009;10:414-9. [Crossref] [PubMed]
- Ibrahim el-SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques--pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson 2011;13:36. [Crossref] [PubMed]
- Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009;11:55. [Crossref] [PubMed]
- Swoboda PP, Larghat A, Zaman A, Fairbairn TA, Motwani M, Greenwood JP, Plein S. Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J Magn Reson Imaging 2014;39:887-94. [Crossref] [PubMed]
- Fairbairn TA, Steadman CD, Mather AN, Motwani M, Blackman DJ, Plein S, McCann GP, Greenwood JP. Assessment of valve haemodynamics, reverse ventricular remodelling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: a cardiovascular magnetic resonance study. Heart 2013;99:1185-91. [Crossref] [PubMed]
- Carasso S, Mutlak D, Lessick J, Reisner SA, Rakowski H, Agmon Y. Symptoms in severe aortic stenosis are associated with decreased compensatory circumferential myocardial mechanics. J Am Soc Echocardiogr 2015;28:218-25. [Crossref] [PubMed]
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007;115:2856-64. [Crossref] [PubMed]
- Attias D, Macron L, Dreyfus J, Monin JL, Brochet E, Lepage L, Hekimian G, Iung B, Vahanian A, Messika-Zeitoun D. Relationship between longitudinal strain and symptomatic status in aortic stenosis. J Am Soc Echocardiogr 2013;26:868-74. [Crossref] [PubMed]
- Hoit BD. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011;4:179-90. [Crossref] [PubMed]
- Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol 2002;283:H792-9. [Crossref] [PubMed]
- Becker M, Kramann R, Dohmen G, Lückhoff A, Autschbach R, Kelm M, Hoffmann R. Impact of left ventricular loading conditions on myocardial deformation parameters: analysis of early and late changes of myocardial deformation parameters after aortic valve replacement. J Am Soc Echocardiogr 2007;20:681-9. [Crossref] [PubMed]
- Mahmod M, Bull S, Suttie JJ, Pal N, Holloway C, Dass S, Myerson SG, Schneider JE, De Silva R, Petrou M, Sayeed R, Westaby S, Clelland C, Francis JM, Ashrafian H, Karamitsos TD, Neubauer S. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging 2013;6:808-16. [Crossref] [PubMed]
- Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J 2002;23:1417-21. [Crossref] [PubMed]
- Brown ML, Pellikka PA, Schaff HV, Scott CG, Mullany CJ, Sundt TM, Dearani JA, Daly RC, Orszulak TA. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2008;135:308-15. [Crossref] [PubMed]
- Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino-Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T. CURRENT AS Registry Investigators. Initial Surgical Versus Conservative Strategies in Patients With Asymptomatic Severe Aortic Stenosis. J Am Coll Cardiol 2015;66:2827-38. [Crossref] [PubMed]
- Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging 2012;5:719-25. [Crossref] [PubMed]
- Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2012;13:827-33. [Crossref] [PubMed]
- Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA, Kanagala P, Masca NG, Clarysse P, McCann GP. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015;41:1129-37. [Crossref] [PubMed]


