Neonatal cranial sonography: ultrasound findings in neonatal meningitis—a pictorial review
Introduction
Late onset neonatal sepsis often presents as neonatal meningitis, which is inflammatory response cerebrospinal fluid and pia-arachnoid infection. High clinical suspicion, prompt diagnosis, immediate institution of therapy and early recognition and management of complications can make a huge difference in the neurological outcome as well as decreased mortality (1). Clinically it can be challenging to evaluate the severity, exact location, and degree of insult to the brain. Cranial sonography (CRS) is of utmost importance in evaluation of sick neonates at bedside due to its portability and is an excellent screening tool due to its non-ionizing properties. CRS has high accuracy in evaluating initial signs as well as complications of bacterial meningitis (2). MRI provides excellent image quality, but its clinical use in NICU’s is currently limited because of costs, logistic and safety issues. MRI and ultrasound are clearly complementary techniques. CT scan is often used in a minor number of cases due to its disadvantages, including but not limited to ionizing radiation and need for sedation.
Discussion
Technique
CRS is performed using a high frequency transducer often through an anterior fontanelle approach. Evaluation of infratentorial structures is limited with anterior fontanelle approach, due to their location away from the transducer, as well as due to inadequate assessment by echogenic tentorium. Alternative acoustic windows include posterolateral (mastoid) fontanelle approach, lateral (temporal) fontanelle approach, lambdoid (posterior) fontanelle, and foramen magnum approach. Images should be symmetrical and should be obtained in at least five coronal and five sagittal planes. The depth gain and time gain compensation (TGC) setting should be adjusted accordingly in the initial images to produce an image filling the sector, containing cranial contours. All precautions must to be made to avoid acquiring too bright or too dark images. Evaluation of superior sagittal sinus is performed using a high frequency linear probe in a coronal plane via the anterior fontanel. If this should fail, the amount of pressure, on the fontanelle, should be reduced. High frequency linear probe is of utmost importance for detailed visualization of superficial structures (meninges, arachnoid and subdural space, cortex) and also tangential vessels in the subarachnoid space (3). Color Doppler interrogation is helpful in various clinical scenarios, especially when screening for patency of intracranial vessels. These things should be kept in mind while performing color Doppler, image should be magnified, color gain should be adjusted to decrease the artifacts from tissues and to increase the signals from vessels. The color box should be limited to the area of interest. Flow velocities and flow indices in intracranial vessels can be evaluated. Power Doppler should be used to interrogate smaller vessels where direction of flow can be ignored.
Etiopathogenesis
Bacteremia leads to CNS infection via seeding of choroid plexus, resulting in infection of CSF and subsequently ventriculitis (Table 1). This is usually followed by infection of the pia and arachnoid mater including the vessels traversing the subarachnoid space. The inflammatory response involves phagocytic cells and inflammatory cytokines (IL-1, IL-6 and TNF-α), culminating in increased membrane and vessel wall permeability, causing formation and accumulation of inflammatory exudates within the sulci (4). Sonographically, increased gyral echogenicity seen in cases of parenchymal involvement is a direct result of arachnoiditis and subependymal gliosis, and can be encountered in the early phases of neonatal meningitis (5).
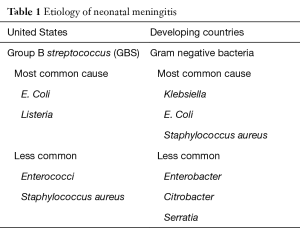
Full table
Sonographic findings (Table 2)
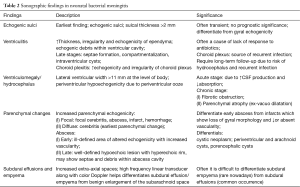
Full table
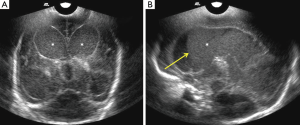
Normal scan
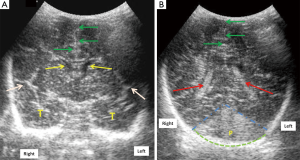
CRS may be normal in some early mild cases (Figures 1,2). In these cases, diagnosis is solely made on clinical and CSF findings.
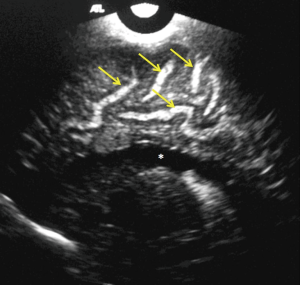
Echogenic sulci
Echogenic sulcus is a very subjective finding and is based on level of competence and experience of sonographer. This is the earliest and the most common finding in neonatal meningitis (Figure 3), which is due to increased thickness as well as echogenicity of the sulci (5,6). Sulcal thickness includes pia and arachnoid mater and should not normally be greater than 2 mm. Widening and increased echogenicity is due to accumulation of inflammatory exudate within the sulci, especially around the pia and arachnoid vessels (5). Occasionally, sulcal echogenicity can be a transient finding and can disappear with clearing of exudates and often are not prognostic (2). It is penultimate to differentiate increased gyral echogenicity, seen in meningitis with parenchymal abnormalities, from echogenic sulci, as parenchymal changes carry poor prognostic implications (5).
Ventriculomegaly
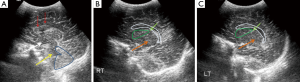
Enlargement of ventricles or ventriculomegaly occurs during acute phases of bacterial meningitis due to increased production as well as decreased CSF absorption, with resultant increased intraventricular pressure. Size of ventricles is evaluated either by using qualitative grading (7) or by measuring the mean width of lateral ventricle, at the level of body of the lateral ventricle, which is normal up to 11 mm in a full term neonate (8). Ventricular size can be divided mild, moderate, marked ventriculomegaly (Figure 4).
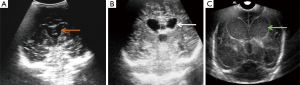
In acute settings, inflammatory exudate increases the intraventricular volume and pressure, hampering normal absorption of CSF by arachnoid villi. In chronic stages, ex-vacuo hydrocephalus is seen in patients with diffuse parenchymal atrophy and subsequent cerebral atrophy. Ependymitis and choroid plexitis also adds to increased CSF production. In next 5–7 days, neutrophils in the exudates are replaced by fibroblasts accelerating formation of septations and intraventricular compartmentalization with resultant CSF outflow obstruction, most commonly at aqueduct of Sylvius and foramen of Luschka and Magendie (9). In chronic phases, ventricular enlargement occurs predominantly due to parenchymal atrophy, often with normal intraventricular pressure. CRS is an easy and reliable tool for detection of ventriculomegaly and can help to ascertain the level of obstruction (5,10). Ventriculomegaly may be reversible in the acute setting; however, there are higher chances of progression, if there are associated parenchymal abnormalities.
Ventriculitis
Sonographically ventriculitis is identified as increased thickness, irregularity and increased echogenicity of the ependyma (Figure 6). Low level internal echoes within the ventricular cavity represent intraventricular inflammatory exudates (Figure 7). Ependymal irregularity occurs due to denudation of small segments of ependyma, resulting in segmental glial proliferation. In long standing cases, the inflammatory exudate may undergo organization into strands and fibrous septae (Figure 6). Septae are composed of denuded and detached segments of ependyma and can result in intraventricular compartmentalization and as a consequence formation of intraventricular cysts and obstructive hydrocephalus (11). Increased echogenicity may also be seen in the periventricular region, due to subependymal infiltration of the inflammatory cells. Inflammation of the choroid plexus presents sonographically as increased echogenicity and irregularity. Clinically, ventriculitis and choroid plexitis can be the underlying cause of lack of adequate response to antibiotic therapy (12). This occurs because ventricles and the choroid plexus can serve as reservoirs of infection and may harbor bacteria even when lumbar puncture yields sterile cultures. Due to risk for recurrent infection, patients with ventriculitis and choroid plexitis are recommended to undergo prolonged follow up as there is a risk for hydrocephalus and increased morbidity (9).

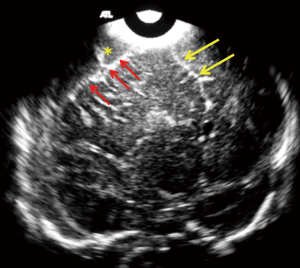
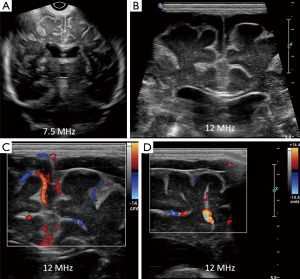
Extra axial fluid collection (EAFC)
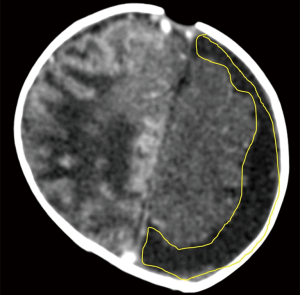
Normally, the brain gyral surface over the convexities is not seen on sonography and the interhemispheric fissure is demonstrated as a linear echogenic structure (2). EAFC displaces the brain away from the vault so sonogram reveals detailed characterization of gyri, midline shift with falx deviation to the opposite side, and compression of ipsilateral lateral ventricle and dilatation of contralateral lateral ventricle (Figure 8). Internal echoes within EAFC signify empyema in the setting of bacterial meningitis (8). Subdural effusion is a common finding in infants with H. influenzae meningitis. High frequency linear ultrasound probe (12 MHz) should always be used in evaluation for EAFC, with color Dopple plays an important role in differentiating subdural effusions from benign enlargement of subarachnoid spaces (Figure 5). Rarely epidural empyema may also be seen (13). EAFC may be missed on while using a low frequency transducer (14). Small EAFC are better delineated on CT or MRI (Figure 9).
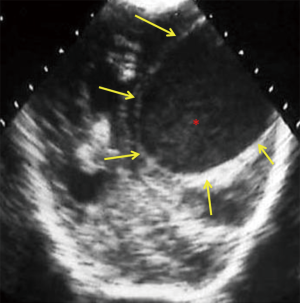
Brain parenchymal findings
Parenchymal changes are seen due to penetration of the organisms from blood vessels into surrounding brain parenchyma (15). The vasculitis that ensues, causes infarction and subsequent liquefactive ischemic necrosis, particularly involves the white matter (2).
Sonographically, parenchymal changes are seen as areas of altered or increased echogenicity which may be diffuse as in cerebritis or focal as in infarction, abscess formation, hemorrhage or focal cerebritis (2,16). Cerebritis is usually the earliest manifestation of parenchymal brain infection. Unsuccessful treatment of cerebritis leads to abscess formation in advanced stages. Abscess formation in early stages can be seen as an ill-defined area of altered echogenicity along with increased vascularity on color Doppler interrogation (2). In advanced stages, it is seen as a well- defined hypoechoic area with a hyperechoic rim (Figure 10). In some cases, there may be hyperechoic septae and echogenic debris seen within the abscess cavity. It is crucial to differentiate early abscesses from ischemic lesions, as the sonographic appearance can be very similar; ill-defined area of altered echogenicity, perilesional edema and mass effect may be seen in both cases. Apart from clinical correlation, an important differentiating feature on sonography is loss of gyral morphology due to associated vasogenic and cytotoxic edema in case of infarcts, along with decreased or absent vascularity within a vascular territory. On serial follow-up, infarcts may demonstrate gradual return of pulsations and development of cystic changes (17). Important differential diagnoses include cystic neoplasm, periventricular and arachnoid cysts, and porencephalic cysts (17,18).
Acknowledgements
The authors would like to acknowledge Dr. Shabnam Bhandari Grover, Department of Radiology and Imaging, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India for providing them with Figure 9.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The study was partly conducted at Kalawati Saran Children’s Hospital, New Delhi, India and partly at Saint Vincent’s Medical Center, Bridgeport, CT.
References
- Tack DM, Holman RC, Folkema AM, Mehal JM, Blanton JD, Sejvar JJ. Trends in encephalitis-associated deaths in the United States, 1999-2008. Neuroepidemiology 2014;43:1-8. [Crossref] [PubMed]
- Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatr Radiol 2008;38:129-37. [Crossref] [PubMed]
- Rumack C, Wilson J. Chapter 51. In: Rumack CM, Wilson SR, Charboneau JW, et al. editor. Diagnostic ultrasound. 3rd ed. St. Louis, MO: Mosby, 2005:1624-5.
- Ye Q, Shao WX, Shang SQ, Shen HQ, Chen XJ, Tang YM, Yu YL, Mao JH. Clinical Value of Assessing Cytokine Levels for the Differential Diagnosis of Bacterial Meningitis in a Pediatric Population. Medicine (Baltimore) 2016;95:e3222. [Crossref] [PubMed]
- Rosenberg HK, Levine RS, Stoltz K, Smith DR. Bacterial meningitis in infants: sonographic features. AJNR Am J Neuroradiol 1983;4:822-5. [PubMed]
- Jéquier S, Jéquier JC. Sonographic nomogram of the leptomeninges (pia-glial plate) and its usefulness for evaluating bacterial meningitis in infants. AJNR Am J Neuroradiol 1999;20:1359-64. [PubMed]
- Han BK, Babcock DS, McAdams L. Bacterial meningitis in infants: sonographic findings. Radiology 1985;154:645-50. [Crossref] [PubMed]
- Baruah D, Gogoi N, Gogoi RK. Ultrasound evaluation of acute bacterial meningitis and its sequale in infants. Indian J Radiol Imaging 2006;16:553-8. [Crossref]
- Mactier H, Galea P, McWilliam R. Acute obstructive hydrocephalus complicating bacterial meningitis in childhood. BMJ 1998;316:1887-9. [Crossref] [PubMed]
- Theodoridou K, Vasilopoulou VA, Katsiaflaka A, Theodoridou MN, Roka V, Rachiotis G, Hadjichristodoulou CS. Association of treatment for bacterial meningitis with the development of sequelae. Int J Infect Dis 2013;17:e707-13. [Crossref] [PubMed]
- Nzeh D, Oyinloye OI, Odebode OT, Akande H, Braimoh K. Ultrasound evaluation of brain infections and its complications in Nigerian infants. Trop Doct 2010;40:178-80. [Crossref] [PubMed]
- Mongkolrattanothai K, Ramakrishnan S, Zagardo M, Gray B. Ventriculitis and choroid plexitis caused by multidrug-resistant Nocardia pseudobrasiliensis. Pediatr Infect Dis J 2008;27:666-8. [Crossref] [PubMed]
- Uchida Y, Matsubara K, Wada T, Oishi K, Morio T, Takada H, Iwata A, Yura K, Kamimura K, Nigami H, Fukaya T. Recurrent bacterial meningitis by three different pathogens in an isolated asplenic child. J Infect Chemother 2012;18:576-80. [Crossref] [PubMed]
- Lowe LH, Bailey Z. State-of-the-art cranial sonography: Part 1, modern techniques and image interpretation. AJR Am J Roentgenol 2011;196:1028-33. [Crossref] [PubMed]
- Foreman SD, Smith EE, Ryan NJ, Hogan GR. Neonatal Citrobacter meningitis: pathogenesis of cerebral abscess formation. Ann Neurol 1984;16:655-9. [Crossref] [PubMed]
- Chowdhary V, Gulati P, Sachdev A, Mittal SK. Pyogenic meningitis: sonographic evaluation. Indian Pediatr 1991;28:749-55. [PubMed]
- Hernanz-Schulman M, Wendy Cohen W, Genieser NB. Sonography of Cerebral Infarction in Infancy. AJNR 1988;9:131-6. [PubMed]
- Raju VS, Rao MN, Rao VS. Cranial sonography in pyogenic meningitis in neonates and infants. J Trop Pediatr 1995;41:68-73. [Crossref] [PubMed]


