Mixed vascular nevus syndrome: a report of four new cases and a literature review
Introduction
The term mixed vascular nevus (or nevus vascularis mixtus) refers to the admixture of cutaneous vascular malformations of the telangiectatic type and angiospastic spots of nevus anemicus (1). These are vascular nevi of the capillary type (1-4). Telangiectatic nevi are congenital skin lesions formed by small, dilated blood capillaries measuring 0.5–1 mm in diameter; these lesions may show areas of normal tissue within the affected area, intermingled with involved areas, leading to a somewhat reticular pattern with preserved irregular borders (2-4). The so-called nevus anemicus represents a vasoconstrictive counterpart of telangiectatic nevi; typically, it is a pale macule with a highly irregular margin. If the skin is rubbed, there is no erythematous reflex within the nevus (1-4). Both nevi may occur as pure cutaneous traits (1-6) or as a part of several genetic skin disorders (6) including phacomatosis pigmentovascularis (PPV) (e.g., phacomatosis cesioflammea and cesioanemica) (7-10) and a newly reported phenotype known as mixed vascular nevus (nevus vascularis mixtus) (1,6,10-12).
Mixed vascular nevus (nevus vascularis mixtus)
This complex dermatologic vascular malformation was initially recognized and comprehensively described by Hamm and Happle in 1986 (1), who reported their personal experience in 4 cases (one child and 3 young adults) and summarized 28 cases (aged 1 to 49 years) that were collected from the literature from 1909 to 1952 (1). They first hypothesized (1) that the combination of these vascular nevi (originally described as “telangiectatic nevus” and its “angiospastic part” (i.e., the nevus anemicus), situated directly adjacent one to each other, could not be regarded as an incidental finding but represented a distinct entity and named it “naevus vascularis mixtus” (1,6). In subsequent reports, Happle (13,14) called these lesions “vascular twin nevi”. In his latest classifications of the various archetypical shapes and patterns of cutaneous nevi, Happle (2-4) categorized the telangiectatic nevus (as well as the nevus anemicus) into the configurations called “patches with indented borders” and “blocks or flags” stating that “lesions adopting these shapes may show areas of normal tissue within the patch, intermingled with involved areas, leading to a somewhat reticular pattern with preserved irregular borders” (2-4). Di Lernia et al. (5) and Happle (14) further defined this skin phenotype understanding that it was characterised by a true admixture of reticular telangiectatic lesions and angiospastic spots of nevus anemicus.
Mixed vascular nevus syndrome
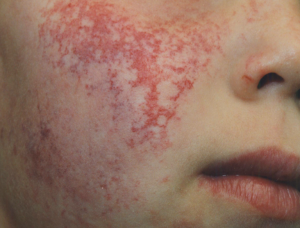
In 2012, some of us (12) reported two individuals with a combination of these paired cutaneous vascular malformations (of the reticular telangiectaticus nevus and angiospastic nevus anemicus types) (nevus vascularis mixtus or paired vascular twin nevi) (Figure 1) coupled with brain abnormalities of the Dyke-Davidoff-Masson type (i.e., crossed cerebral/cerebellar hemiatrophy with hypoplasia of the ipsilateral cerebral vessels and homolateral hypertrophy of the skull and sinuses (hyperpneumatisation) with contralateral hemispheric hypertrophy) (15) in association with systemic abnormalities consisting in facial asymmetry, skeletal anomalies (i.e., Legg-Calvé-Perthes-like disease) and disorders of autoimmunity (e.g., diabetes, thyroiditis).
In 2014, Happle (10) proposed to name this complex malformation phenotype with the eponym Ruggieri-Leech syndrome melting the first report where the pure paired cutaneous trait (i.e., mixed vascular nevi) was associated to extra-cutaneous manifestations (10) to a previous report by Leech et al. (11), who coupled a capillary malformation identical to the mixed vascular nevus (but not yet understood and/or labelled as nevus vascularis mixtus) to a brain malformation of the megalencephaly type (i.e., similar to the phenomena of increased growth seen in the Dyke-Davidoff-Masson syndrome).
Methods
Patients
Patients were selected from the database of neurocutaneous disorders at the University of Catania. Each patient underwent full clinical and laboratory examination, including general, dermatological, ophthalmological and neurological examination and full laboratory investigation including ECG, heart ultrasound, abdominal and pelvic ultrasound examination, standard- and video-EEG, and X-ray films when dictated by clinical findings. All patients underwent magnetic resonance imaging studies of the central nervous system using Signa-HDdxt 1.5 Tesla (G.E. Healthcare, Milwaukee, USA) with T1, T2, T2-FLAIR, and MRA sequences. CT scans were obtained using Optima CT660 (G.E. Healthcare, Milwaukee, USA) with spiral multi-slice technique. The local ethics committee (Comitato Etico 1, University of Catania) approved this retrospective analysis and reporting. Patients’ consent was obtained (and/or waived due to the non-interventional retrospective analysis nature of this study).
Literature review
Studies published in the literature (Medline, Scopus, WOS and bibliographies) reporting on the coexistence of nevus anemicus and nevus telangiectaticus in association or not with neurological involvement and mixed vascular nevus syndrome were reviewed (all years). Literature search was performed by using the following key words: “nevus anemicus”, “nevus telangiectaticus”, “nevus vascularis mixtus”, “mixed vascular nevus syndrome”, “Dyke-Davidoff-Masson syndrome”, “neurological”, “nervous system”, and “MRI”: were considered studies reporting data in English, German, French and Spanish.
Results
Patients
We identified 4 patients (3 males, 1 female; currently aged 2 to 34 years; median age =13.75 years) who had the coexistence of nevus anemicus and nevus telangiectaticus (mixed vascular nevi) in association with extra-cutaneous features including neurological and systemic manifestations first seen and followed-up (mean follow-up =8 years) at the University of Catania. The main clinical, laboratory and imaging findings are summarised in Table 1 and illustrated in Figures 2,3,4.
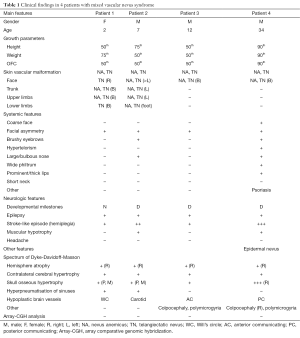
Full table
Literature review
Literature review revealed 110 results whose clinical reports contained information regarding the coexistence of nevus anemicus and nevus telangiectaticus. Only 4 out of these 110 studies (1,6,10,13) contained information regarding the spatial and temporal admixture of a mixed vascular nevus in association (10,13) or not (1,6) with extra-cutaneous features. Hamm and Happle (1) collected information on 28 cases (aged 1 to 49 years) from the literature and personally examined four additional cases. We identified 2 studies (10,13), including our previous study (10) on two patients with mixed vascular nevus syndrome (currently still under follow-up at our Institutions) for a total of 3 patients (2 M, 1 F) with mixed vascular nevus syndrome [see also reference (12)].
Mixed vascular nevus syndrome: definition of the neurocutaneous phenotype
By literature review (1,6,10-12) and personal experience [including data reported in Table 1 and follow-up of patients reported in reference (12)] we aimed to better define the cutaneous and extra-cutaneous phenotype of the mixed vascular nevus syndrome as follows.
Cutaneous features
Co-occurrence of telangiectatic nevus and nevus anemicus situated directly adjacent one to each other (spatial and temporal admixture) (Figure 1). Notably, by literature review and personal experience we recorded these two dermatologic traits adjacent or in close proximity one to each other or in distant areas with satellite macules (Figure 1): however, it is the contemporary presence in the same individual the skin hallmark of disease.
Typically, enhancement of the paler inner-dotted areas and satellite macules is usually demonstrated upon stroking (or applying heat or cold) to the nevus anemicus and adjacent skin, which became erythematous. Histological analysis from affected area shows wider capillaries’ lumens lined with flat endothelial cells, without an increase in melanin pigmentation.
Neurological phenotype
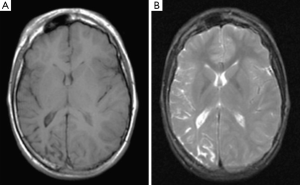
There were mild to moderate developmental delay with cognitive deficits and behavioural abnormalities (usually hyperactivity and opposing behaviour), crossed alternating episodes of simple partial and/or hemiplegic seizures, and speech difficulties. There is no progression. Seizures, which in some cases, could be regarded as stroke-like episodes, are either secondary to the occasional vasoconstriction of the angiospastic/hypoplastic brain vascular vessels (see below), or, more frequently, secondary to the underlying cortical brain malformation. Regardless to their nature and semiology, these paroxysmal events become less frequent after adolescence (unless associated to moderate to severe brain malformations: see also Figure 4 and below).
Systemic abnormalities
These consist of (mild to moderate) facial asymmetry with facial dysmorphic features (e.g., rounded and coarse face with synophris, hypertelorism, a large and bulbous nose, a large philtrum with thin lips, and short neck), dental anomalies including irregularly shaped and malpositioned teeth, skeletal anomalies (i.e., Legg-Calvé-Perthes-like disease) and disorders of autoimmunity including diabetes and thyroiditis (12).
Brain imaging abnormalities
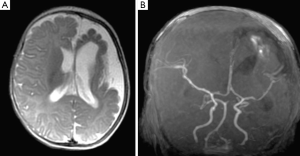
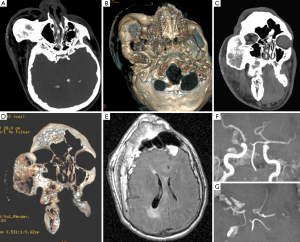
Brain computerised tomography (CT) and/or magnetic resonance imaging (MRI) usually reveals (Figures 2-4) asymmetry of the cerebral hemispheres with mild (Figure 3A,B) to moderate (Figure 4E,F) or severe (Figure 2A) hemispheric atrophy; ipsilateral osseous hypertrophy (Figures 3,4); hyperpneumatisation of the paranasal sinuses (and mastoid cells) (12) and contralateral cerebral hypertrophy (Figure 3). The cerebral circle is usually affected, ranging from mild (Figure 2) to moderate (Figure 4) degrees of dysplasia, including hypoplastic hemi-circle of Willis with hypoplasia of P1 and A1 cerebral arteries (Figures 2B,4F,G). The spectrum of brain abnormalities may include malformations of cortical development (Figures 2,3,4) with megalencephaly (Figure 3), dilated ventricles (colpocephaly), polymicrogyria and pachygyria (Figures 2A,4E) (11,12). The brain atrophy and ipsilateral vascular hypoplasia could be minimal (12). The degree of osseous hypertrophy could be impressive and extended to the frontal, maxillary and mandibular regions simulating an osseous dysplasia (Figure 4A-D) with somewhat dotted calcification of the soft tissues in the affected area with the cutaneous vascular malformation (Figure 4A-D) (Table 1).
Single photon emission computerized tomography (SPECT) study of the brain showed in one case (12) reduced blood perfusion in the atrophic brain hemisphere with normal flow in the middle portion of the frontal and occipital lobes.
Overlapping features and misdiagnoses with other neurocutaneous disorders associated to vascular malformations
The so-called PPV (7,10,16) is defined as the coexistence of a widespread vascular (usually capillary) nevus (nevus flammeus) and an extensive pigmentary nevus (usually of the Mongolian spot type or blue/slate/grey oculo-cutaneous melanocytosis) associated or not to a variety of other cutaneous nevi including nevus anemicus, nevus telangiectaticus, epidermal nevus, nevus spilus or cutis marmorata telangectasica and/or extra-cutaneous alterations. The various combinations of skin nevi are currently classified as (7): (I) PPV type I (capillary malformation + epidermal nevus; (II) PPV type II (type IIa: nevus flammeus and Mongolian spot; type IIb: IIa + nevus anemicus; (III) PPV type III (type IIIa: nevus flammeus and nevus spilus; type IIIb: type IIIa + nevus anemicus; (IV) PPV type IV (nevus flammeus and Mongolian spot and nevus spilus + nevus anemicus; (V) PPV type V (Mongolian spot and cutis marmorata telangiectatica congenita. An alternative classification (9) is based on five subtypes only classified according to the colour of nevi and follows the Latin nomenclature: (I) phacomatosis cesioflammea (caesius = blue grey of the Mongolian spot plus the nevus flammeus: i.e., identical to the traditional types IIa and IIb); (II) phacomatosis spilorosea (nevus spilus coexisting with a pale-pink telangiectatic nevus (nevus roseus, which is of a much lighter hue than nevus flammeus): i.e., corresponding to the traditional types IIIa and IIIb); (III) phacomatosis melanorosea (large café-au-lait spots coexisting with nevus roseus sometimes accompanied by cutis marmorata-like lesions); (IV) phacomatosis cesiomarmorata (blue spots and cutis marmorata telangiectatica: i.e., a descriptive term for PPV type V); and (V) phacomatosis cesioanemica (blue nevus coexisting with nevus anemicus): i.e., a variant of phacomatosis cesioflammea. Associated features involve the eye (melanosis bulbi, iris mammillations, megalocormea) and musculoskeletal (facial and limb overgrowth, macrocephaly and scoliosis) systems. As outlined above, phacomatosis cesioflammea and cesioanemica (2,17,18), which encompass within their phenotypes nevus anemicus and variants of nevus flammeus could be misdiagnosed as mixed vascular nevus syndrome, unless their pigmentary counterparts (i.e., blue-grey nevi of the Mongolian type) are well recognised.
Pathogenesis
A unifying didymotic theory: allelic vs. non-allelic twin spotting phenomena
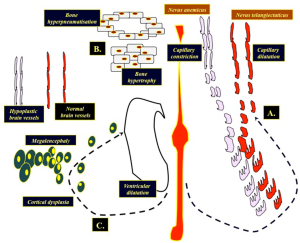
Analogously to what occurs in other organisms, these phenotypes suggest that pairing and somatic recombination of recessive (didymotic) alleles controlling the balance between constriction (i.e., nevus anemicus) and dilatation (i.e., nevus telangiectaticus) of cutaneous blood vessels in an embryo could be the primary event causing twin spotting (i.e., paired vascular twin nevi) (Figure 1) and the appearance of the two neighbouring and partially admixed cell populations would be the secondary phenomenon (2-5,10). In addition to that, similarly to what occurs in the skin, the crossed hemi-cerebral malformation (Figures 2,3,4) may well represent dichotomic functional abnormalities likely reflecting allelism at the same, yet undiscovered, underlying gene loci of the vascular twin nevi (Figure 5). By taking into consideration all these events, we propose a unifying didymotic theory to explain the pathogenesis of cutaneous and extra-cutaneous phenomena in mixed vascular nevus syndrome: the dichotomic phenomena of capillary dilatation (in the skin and brain vessels), hyperpneumatisation of the sinuses (in the skull) and telangiectasia/ventriculomegaly vs. capillary constriction (in the skin and brain vessels, see Figure 5A), bone hypertrophy (in the skull, see Figure 5B) and cortical dysplasia/megalencephaly (see Figure 5C) may reflect paired phenomena affecting different tissues (1,19).
Management
Cosmetic facial alterations caused by the mixed vascular malformations can best be treated by sophisticated laser therapeutic techniques. The results are satisfactory in cases with capillary malformations of low pigmentation (12), with lightening of the pigmented zone [see case 2, reference (12)].
Seizures can be treated similarly to those in patients without mixed vascular nevus syndrome. Carbamazepine or oxicarbamazepine, valproic acid, topiramate, levitiracetam, hydantoins and clonazepam are the most used antiepileptic drugs (6,12). Patients with intractable seizures, benefit of combination of antiepileptic drugs. Low-dose aspirin (3–5 mg/kg/d) is used to optimize stroke-like episodes and neurodevelopmental outcome, as in Sturge-Weber syndrome, with minimal side effects (6,12,20).
Scoliosis (and/or other orthopaedic anomalies) and spasticity, when present, have been treated with physiotherapy, orthopaedic help or botulism toxin.
Problems at school that affect intellectual function and behaviour in most cases, may need special help and stimulants drugs, such as methylphenidate, associated with antiepileptic treatment.
Paired vascular twin nevi of the telangiectatic and anemicus types are likely more frequent than previously thought and should be investigated by means of: (I) brain and spinal cord imaging (combination of CT and MRI studies); (II) skeletal X-ray studies (when dictated by clinical findings); (III) systemic ultrasound studies; (IV) neurophysiologic studies (EEG); (V) psychomotor testing; (VI) and laboratory investigation (including immune-mediated dysfunction).
Conclusions
The nevi pigmentation is stable and the skin within the nevi area is firm over years: it can occasionally become dry and pruriginous. Overall, the skin complexion and texture are different from other vascular malformations syndromes (e.g., Sturge-Weber syndrome) without progression or hypertrophy of the soft tissues.
In our experience and after long-term follow-up, seizures and/or stroke-like events, regardless to their nature and semiology, become less frequent after adolescence (unless associated to complex underlying nervous system malformations). The psychomotor outcome is fine with good social integration. Brain imaging follow-up reveals no progression or vascular degeneration.
The autoimmune skin phenomena usually decrease with age and the immune-mediated systemic abnormalities respond to usual treatments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee (Comitato Etico 1, University of Catania). Patients’ consent was obtained (and/or waived due to the non-interventional retrospective analysis nature of this study).
References
- Hamm H, Happle R. Mixed vascular nevus. Report of 4 cases. Hautarzt 1986;37:388-92. [PubMed]
- Happle R. editor. Mosaicism in Human Skin. Understanding Nevi, Nevoid Skin Disorders, and Cutaneous Neoplasia. Berlin/Heidlber: Springer-Verlag, 2014.
- Happle R. Capillary malformations: a classification using specific names for specific skin disorders. J Eur Acad Dermatol Venereol 2015;29:2295-305. [Crossref] [PubMed]
- Happle R. The categories of cutaneous mosaicism: A proposed classification. Am J Med Genet A 2016;170A:452-9. [Crossref] [PubMed]
- Di Lernia V, Valeri F, Patrizi A. What is your diagnosis? Naevus vascularis mixtus. Ann Dermatol Venereol 1990;117:469-70. [PubMed]
- Ruggieri M, Praticò AD. Mosaic Neurocutaneous Disorders and Their Causes. Semin Pediatr Neurol 2015;22:207-33. [Crossref] [PubMed]
- Enjolras O, Mulliken JB. Vascular malformations. In: Harper J, Oranje A, Prose N. editors. Textbook of Dermatology. Oxford: Blackwell Science, 2000:975-6.
- Hasegawa Y, Yasuhara M. Phakomatosis pigmentovascularis type IVa. Arch Dermatol 1985;121:651-5. [Crossref] [PubMed]
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol 2005;141:385-8. [Crossref] [PubMed]
- Happle R. Didymotic Skin Disorders. In: Happle R. editor. Mosaicism in Human Skin. Understanding Nevi, Nevoid Skin Disorders, and Cutaneous Neoplasia. Berlin/Heidlber: Springer-Verlag, 2014:109-14.
- Leech SN, Taylor AE, Ramesh V, Birchall D, Ann Lynch S. Widespread capillary malformation associated with global developmental delay and megalencephaly. Clin Dysmorphol 2004;13:169-72. [Crossref] [PubMed]
- Ruggieri M, Milone P, Pavone P, Falsaperla R, Polizzi A, Caltabiano R, Fichera M, Gabriele AL, Distefano A, De Pasquale R, Salpietro V, Micali G, Pavone L. Nevus vascularis mixtus (cutaneous vascular twin nevi) associated with intracranial vascular malformation of the Dyke-Davidoff-Masson type in two patients. Am J Med Genet A 2012;158A:2870-80. [Crossref] [PubMed]
- Happle R. Loss of heterozygosity in human skin. J Am Acad Dermatol 1999;41:143-64. [Crossref] [PubMed]
- Happle R. Dohi Memorial Lecture. New aspects of cutaneous mosaicism. J Dermatol 2002;29:681-92. [Crossref] [PubMed]
- Arora R, Rani JY. Dyke-Davidoff-Masson syndrome: imaging features with illustration of two cases. Quant Imaging Med Surg 2015;5:469-71. [PubMed]
- Hall BD, Cadle RG, Morrill-Cornelius SM, Bay CA. Phakomatosis pigmentovascularis: Implications for severity with special reference to Mongolian spots associated with Sturge-Weber and Klippel-Trenaunay syndromes. Am J Med Genet A 2007;143A:3047-53. [Crossref] [PubMed]
- Vidaurri-de la Cruz H, Tamayo-Sánchez L, Durán-McKinster C, Orozco-Covarrubias Mde L, Ruiz-Maldonado R. Phakomatosis pigmentovascularis II A and II B: clinical findings in 24 patients. J Dermatol 2003;30:381-8. [Crossref] [PubMed]
- Fernández-Guarino M, Boixeda P, de Las Heras E, Aboin S, García-Millán C, Olasolo PJ. Phakomatosis pigmentovascularis: Clinical findings in 15 patients and review of the literature. J Am Acad Dermatol 2008;58:88-93. [Crossref] [PubMed]
- Restivo DA, Milone P. Teaching NeuroImages: progressive facial hemiatrophy (Parry-Romberg syndrome) with ipsilateral cerebral hemiatrophy. Neurology 2010;74:e11. [Crossref] [PubMed]
- Comi A. Current Therapeutic Options in Sturge-Weber Syndrome. Semin Pediatr Neurol 2015;22:295-301. [Crossref] [PubMed]


