Techniques for respiration-induced artifacts reductions in thoracic PET/CT
Abstract
The advent of positron emission tomography/computed tomography (PET/CT) provides fusion of both anatomical and functional information. CT-based attenuation correction replaced 68Ge-based attenuation correction for shortening acquisition time, improving image quality and quantitative accuracy. However, due to the temporal difference of PET and CT, mis-registration and motion artifacts are observed in the attenuation-corrected images mainly due to the respiratory motion. Reducing the spatial mismatch of the PET and CT reconstructed image remains a challenge. This review provides an introduction to various respiratory image artifacts reduction techniques especially for thoracic lesions, including breathing instruction based methods, CT protocol based methods and 4-dimensional PET/CT. The advantages and drawbacks of different methods are also discussed.
Keywords
PET/CT; respiratory motion; artifacts; attenuation correction
Introduction
Before PET/CT scanners were invented, attenuation correction was performed mostly with an external 68Ge 511 keV source that rotated around the patient. Nowadays, most clinical PET/CT machines no longer equipped with 68Ge-based source. Instead, CT-based attenuation correction was used in the combined PET/CT scanner.
This new design produced huge numbers of photons in the transmission scan even at low tube currents. Hence it is much faster than the 68Ge-based attenuation correction scan and has lower statistical noise level (1,2). Also, CT-based attenuation correction ensures the significant energy difference between the gamma-rays and X-rays, so that the transmission scan would not be contaminated by the emitted gamma-rays from the patients. This leads to a more accurate activity concentration values and better uniformity (3).
On the other hand, CT-based attenuation correction for PET is hampered by artifacts that are usually not seen in the standalone PET images. Examples are artifacts due to respiratory motion (4), truncation (5), metallic implants (6) and CT contrast agents (7). Among all these factors, respiratory motion is generally considered to be the main problem in CTbased attenuation correction. For standalone diagnostic CT, the optimized protocol is to obtain a 3D helical acquisition of the thoracic cavity over a single full-inspiration breath-hold CT scan. When applied with the emission exam, this technique captures a snapshot of the thoracic cavity in a distinct respiratory phase and does not represent the time-averaged position of the thoracic structures as PET acquisition does. This misalignment between transmission and emission scan is most noticeable at the left lung especially for two regions: (I) tissues around lower thorax and upper abdomen, where the transmission scan may not be presented in the emission scan; (II) tissues around the lung and left-ventricle interface, where myocardial uptake is prominent overlying the left lung of the CT in the fused images. In fact, for thoracic structures, more than 40% of the studies have misalignments between the measured and the true position (8). Erdi et al. examined PET/CT images of 5 patients with multiple lung carcinoma lesions, and showed that the spatial mismatch resulted in up to a 30% error in the standardized uptake value (SUV) of the lesions (99). Also, phantom studies showed the effect of motion can result in as much as 75% underestimation of the maximum activity concentrations (10). These distortions may lead to inaccurate localization of tumors and hence potential misdiagnoses (11,12).
Multi-row CT technology that employs 6 or more detector rows can reduce magnitude and frequency of respiration-induced artifacts in PET/CT system (13). This is because, with multirow CT technology, the CT examination time of whole-body PET/CT scan is shortened to 20 s or less. Therefore, patients will be more cooperative for breath-hold during the CT acquisition. Some authors also suggested viewing the emission images with and without the attenuation correction respectively to exclude respiration-induced bias (12).
Still, more effective methods are warranted to reduce the PET/CT respiratory artifacts. Three main categories of these methods have been mostly investigated so far: breathing instruction, CT protocols and 4-dimensional (4D) PET/CT.
Breathing instruction based methods
Optimal breathing patterns
Instead of suggested deep-inspiration breath-hold protocol in diagnostic CT, many researchers believed normal-expiration breath-hold is the best option for transmission scan in thoracic PET/CT (14,15). In a survey performed by Goerres et al. (16), CT scans were performed at four respiratory levels: freebreathing, maximal-inspiration, maximal-expiration and normalexpiration. For different levels, multiple distances between the anatomical landmarks or a reference point in the CT and corresponding PET images were compared. Normal-expiration breath-hold showed best results with better match and smaller range of measured distances in comparison with other breathing patterns for the studies focusing on the upper abdominal organs. Furthermore, this CT protocol reduced the occurrence and the severity of respiration-induced curvilinear artifacts on coregistered PET/CT. Another study showed that reconstructed images from patients of normal-expiration breath-hold group had 28% less incidence rate of artifacts compared with those from the free-breathing group (15). One possible explanation is that a normal human spends most time in expiration in the whole respiratory cycle. Further comparison study showed that normal-expiration CT scan for attenuation correction gave good SUV recovery in the case of mobile tumors with size of 20 mm, even when the magnitude of breathing was large (17). Attenuation correction using maximal-inspiration breath-hold protocol resulted in more serious underestimation of SUV especially when breathing amplitude increased.
The clinical challenge of normal-expiration breath-hold protocol is patient’s compliance and comfort. Many patients are incapable of maintaining breath-hold for the duration of the whole-body CT examination, even for the most advanced multi-row CT scanner. This results in severe compensatory breathing artifacts at the midscan level, i.e., the lower thorax to the upper abdomen region, when the patient resumes breathing during the CT acquisition.
Deep-Inspiration Breath-Hold (DIBH) PET/CT
Nehmeh et al. proposed a deep-inspiration breath hold protocol for both CT and PET (DIBH PET/CT) acquisition (18). The patient was instructed to breathe deeply and then hold the breath, under the monitoring with an amplitude gating device, i.e., the real-time position management (RPM, Varian Medical Systems, Palo Alto, CA). Breath-hold CT data were acquired for about 16 s in the helical mode. PET scan was divided to nine 20 s frames, i.e., 3 mins. In the beginning of each PET frame, patient was instructed to breathe and hold the breath again as in DIBH CT. PET data were acquired for only one FOV. This method showed an increase in lesion SUV of as much as 83% and a reduction in the distance between the centroids of PET and CT lesions of as much as 49%, compared to the conventional breathhold PET/CT. Similar study was proposed by Meirelles et al.(19) and Torizuka et al. (20). They both showed increase in SUV and more precise localization and quantification of lesions when using DIBH PET/CT. Higher diagnostic accuracy of DIBH PET with a single 30 s, 45 s or even 60 s scan can be achieved (21,22).
By now the DIBH method was only explored in lung lesion studies. Additional external monitoring such as RPM and breathecoaching are also needed to measure the respiratory cycle, ensuring the matching of the transmission and the emission respiratory phases. Moreover, even a single breath-hold for 20 s is not acceptable for senior patients or patients who have underlying lung diseases such as emphysema or pulmonary fibrosis (20), let alone multiple breath-holds in DIBH. Unsuccessful breath-hold due to irregular breathing pattern may cause highly variable results, which will affect the diagnostic accuracy.
CT protocal based methods
Specific CT protocols for attenuation correction of PET images have been proposed to replace conventional breath-hold CT. Averaging the CT data over many respiratory cycles ensure that the CT attenuation map matches with the PET, leading to reduction of breathing-induced artifacts such as PET/CT spatial mismatch, target blurring and underestimation of the SUV.
There are several approaches to this averaging protocol, some of which are outlined in this review: slow CT, low-pitch CT in helical mode, cine average CT and interpolated average CT.
Slow CT
Lagerwaard et al. introduced slow CT as a method to average several respiratory cycles over the scan duration (23). The tube rotation time of a single slice CT was slowed down to 4 s/rot. Pitch was set to 1. Compared with the diagnostic deepinspiration breath-hold CT, slow CT was more similar to the average position of the thoracic cavity structures in PET.
In another study from Sorensen et al. (24), severe “gap” artifacts between successive PET reconstructed slices were observed for slow CT-based attenuation correction. It was possibly due to the inconsistencies in the projections from large respiratory motions combined with slow CT propagated into the corrected PET images. Hence, this method may not be appropriate for attenuation correction in PET/CT for large respiratory motion amplitudes.
Low-pitch CT
New generations of CT provide more configurations for other “slow CT” methods. Nye et al. set the pitch as 0.562 which was the lowest value allowed by the scanner (25). This pitch value increased the axial sampling during free-breathing mode without increasing the tube rotation time. A total of 16 s scan was used to cover the chest cavity. Final CT data were matched with the PET slice thickness for attenuation correction and to suppress respiratory motion artifacts. Compared to conventional breathhold CT, this low-pitch CT reduced the number of problematic studies from 71% to 28%.
One concern for low-pitch CT is the radiation burden. Due to the potential long exposure time, high radiation dose for the low-pitch CT is delivered. The radiation dose was approximately 2.3 mSv as compared to a 1.8 mSv of the conventional breathhold CT. The dose length product was 153 mGy, also higher than the conventional method of 117 mGy (25). The relative high radiation dose hampers the low-pitch CT from clinical application.
Cine average CT (CACT)
Pan et al. introduced a CT protocol named 4-dimensional CT (4D CT) for PET/CT attenuation correction (26). 4D CT provided images of all phases of the breathing cycle for tumor staging and radiation therapy treatment planning (27). Cine mode technique acquired repeated axial CT images at each table position for a certain time period. This method produced even thinner slice thickness than those of a low-pitch helical scan. Pan et al. (26) and Cook et al. (28) averaged the images of 10 phases in 4D CT to form a respiratory cine average CT(CACT) at each table position. The average CT of the thorax was then combined with the helical CT data of the tissue outside the thorax, e.g., abdomen, to make up the integrative CT images. This method greatly improved registration and tumor SUV as compared to deep-inspiration and normal-expiration breath-hold helical CT attenuation correction. Similar results were achieved by Gould et al. (8). Alessio et al. further evaluated both average and intensity maximum images of 4D CT for the PET/CT misalignment reduction (29). Fewer misalignments were observed with intensity maximum 4D CT. Moreover, compared to helical CT, 4D CT was more flexible to be retrospectively averaged or processed.
There are mainly two adverse problems for CACT: (I) increasing the CT acquisition time due to the longer period of each bed position; (II) increasing the radiation dose to the patient. Lowering the tube current can potentially reduce the dose by a wide margin (30).Interpolated average CT (IACT)
An alternative method for CACT is interpolated average CT (IACT) to reduce radiation dose with similar image quality (31). CT images of desired phases, e.g., end-inspiration and endexpiration phases from a respiratory cycle, are used to generate the interpolated phases by a deformable image registration method named optical flow method (OFM) (32). The IACT is calculated by averaging the original and interpolated phases. In a clinical study, Huang et al. obtained the IACTs using different number of desired phases from a cine CT (31). The PET images were then reconstructed with attenuation correction using different IACTs. The maximum SUV difference between the use of IACT and CACT was about 3%. The radiation dose using IACT with 2 original phases, i.e., end-expiration and endinspiration could be potentially reduced by 85% as compared to the use of CACT.
A further simulation study showed that IACT was a robust method that worked for maximum respiratory motion amplitude of up to~3 cm (33). The results showed in Figure 1 and Figure 2 that IACT, similar to CACT, provided improved image quality as compared to conventional helical CT.
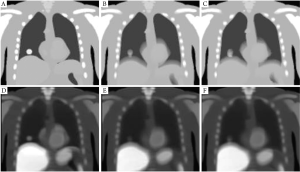
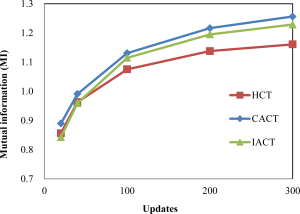
4-Dimensional (4D) PET/CT
Gated PET
Gated PET was firstly proposed for the standalone PET in brain scan to remove patient motions. To reduce smearing due to the breathing motions and improve quantification of 18F-FDG uptake in lung lesions, Nehmeh et al. proposed respiratory gated PET (34). PET data were acquired into discrete bins in synchrony with the breathing cycle. Ten bins data were acquired for a FOV, with 300-500 ms time interval between each 1 minute gated bin. The lesion motion was negligible due to the short acquisition interval and thereby approximately motion-free images were obtained. During the gated PET acquisition, different respiratory tracking systems have been used to monitor the respiratory motion and generate a trigger at predefined amplitude or phase. Studies using this technique showed an improvement in the target-to-background ratio and a more accurate measurement of the SUV (34,35).
Phase matched 4D PET/CT
Instead of manipulating CT protocols to match the PET, phase matched 4D PET/CT applied gated PET images to match specified phase of CT. However, gated PET is seldom directly corrected by a breath-hold CT as their respiratory phases are not synchronized. Hence its reconstructed image quality is even worse than those of the non-gated PET with attenuation correction by a conventional breath-hold CT. In 4D PET/CT imaging, the previously described 4D CT and gated PET are combined (36): the 4D CT images spatially match with the gated PET images. In synchrony with the RPM breathing signal, respiratory gated PET data are acquired into discrete bins. 4D CT data are acquired and sorted according to their phases to generate a respiratory gated CT data. To make the CT images in each phase coincide with the PET images, the gated CT images at each bin are then spatially re-binned and re-sliced in the axial direction. The gated PET data are then corrected for attenuation with the corresponding gated CT data.
In a clinical study, Nehmeh et al. measured distance of the lesion centroids between the gated PET and phase matched 4D CT (36). The result showed an improvement in lesion registration of PET and CT was up to 41% as compared to the registration between gated PET and conventional breath-hold CT. Also, a reduction in PET derived tumor volumes of up to 42%, and an increase in lesion SUV of up to 16% were also found. Similar researches (37-39) all showed that phase matched 4D PET/CT provided superior results as compared to gated PET with a breath-hold CT and non-gated PET with a breathhold CT for attenuation correction.
Algorithm-based 4D PET/CT
One may notice that in 4D PET/CT each PET bin is independent from others after the re-binning process. Usually, each respiratory PET phase only matches well with one corresponding CT phase. Attenuation correction of this respiratory PET phase provides an artifact-free image, since the PET and CT images are coregistered to an identical phase. Reconstructed images, however, have lower counts thus a poor signal-to-noise ratio (SNR).
In principle, to form a PET/CT image, it is better to utilize all PET information from the whole respiratory gated acquisition instead of only one phase. One method to achieve this goal is that before attenuation correction each respiratory PET bin is transformed into one referenced target bin that corresponds optimally to a matched CT phase image from gated CT. Several researchers used the motion vectors calculated from PET to do the transformations, and there are two main categories of the algorithms.
I. Rigid model transform. The movement of the heart due to breathing is difficult to model. It can be approximated by a rigid transformation with simple translational and rotational steps. For cardiac transform, affine linear transform algorithm is a rigid motion transform for shear and compression. Based on the successful cardiac application, Livieratos et al. used this algorithm to reposition the lungs with list-mode projection data before reconstruction (40). The rigid motion was calculated from the simulated CT images of the digitized phantom. Significant respiratory motion compensation in the lungs was observed in comparison with the ones without transformation applied on the list-mode data in the reconstructed images. The improvement of contrast slightly varied for lung lesion of different locations and sizes, and the smaller lesions (7 and 11 mm) suffered from significant partial volume effects.
II. Non-rigid model transform. Lung motion due to respiratory motion is non-rigid in nature. CT attenuation images are not always co-registered to PET images especially at the lower thorax and diaphragm where large deformations often happen. This will lead to inaccurate PET reconstructed images. Thus for lung motion, non-rigid motion correction algorithms would be superior to rigid motion correction algorithms.
Lamare et al. used B-spline model to derive motion vectors from different simulated CT phases (41). Then elastic transformation was incorporated to the list-mode PET raw data. The result showed the elastic transformation led to a more uniform improvement across the lungs for different sizes and locations of the lesions. Thorndyke et al. proposed a retrospective stacking method (42). They used a deformable registration based on a B-spline model to derive the motion vectors from the noisy PET reconstruction data. Retrospective stacking method based on deformable registration yielded reduced blurring and increased SNR in the reconstructed images. Dawood et al. introduced the optical flow method (OFM) to calculate a 3D vector from a PET raw data (43). For gated PET, OFM was further developed for estimating motion vectors between any bin and the target bin. The reconstructed image contained all PET information with minimal motion, leading to more accurate attenuation correction and quantification.
The 4D PET/CT transform algorithm is very computationally intensive. It also requires gating hardware to support. In the meantime, this method may not be feasible for most clinicians because of the high complexity of implementation.
Conclusion
CT-based method is better than the conventional 68Ge-based transmission scan for attenuation correction in many aspects. However, it is hampered by respiration -induced artifacts. Several approaches to correct these artifacts in PET/CT images have been reviewed.
Attenuation correction with breath-hold CT is the easiest way to obtain the reconstructed PET image. Among different breathing patterns, normal-expiration breath-hold CT shows the best result. However, patient compliance is critical to ensure the breath-hold duration for both breath-hold CT and DIBH methods. Some patients with heart disease or lung tumor usually do not have normal cardio-pulmonary function, and it is hard to coach them to hold and release their breath over many cycles during the examination.
With the computational capability of advanced computers, pre- and post-processing techniques are preferred to achieve a better PET/CT registration. Further averaging the CT data over one or many respiratory cycles matches the PET data better. However, PET attenuation correction with slow CT results in severe inconsistent artifacts in the reconstructed image. Lowpitch CT and cine average CT overcome the inconsistent artifacts for PET/CT attenuation correction, while they have the problem of higher radiation dose, which is a significant consideration for patients. An alternative method using IACT for attenuation correction is introduced to further reduce radiation dose with similar image quality of cine average CT.
4D PET/CT is an ongoing research technique to eliminate spatial blurring of the emission data. However, it takes huge amount of efforts for data acquisition and processing. Phase matched and algorithm-based 4D PET/CT may only be feasible for research institutions.
Each respiratory artifact reduction technique has its own advantages and disadvantages and the optimum approach may probably be task- or patient-dependent and remains to be determined. While improving image quality is important, the actual implementation of the respiratory artifact reduction technique highly depends on the robustness and complexity of the clinical set up.
Acknowledgements
This work was supported in parts by the Start-up Research Grant (SRG004-FST11-MSP) and Multi-Year Research Grant (MYRG185(Y1-L3)-FST11-MSP) of University of Macau, Macau.
References
- Kinahan PE, Townsend DW, Beyer T, et al. Attenuation correction for a combined 3D PET/CT scanner. Med Phys 1998;25:2046-53.[LinkOut]
- Nakamoto Y, Osman M, Cohade C, et al. PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation-corrected images. J Nucl Med 2002;43:1137-43.[LinkOut]
- McCord ME, Bacharach SL, Bonow RO, et al. Misalignment between PET transmission and emission scans: its effect on myocardial imaging. J Nucl Med 1992;33:1209-14; discussion 1214-5.[LinkOut]
- Beyer T, Antoch G, Blodgett T, et al. Dual-modality PET/CT imaging: the effect of respiratory motion on combined image quality in clinical oncology. Eur J Nucl Med Mol Imaging 2003;30:588-96.[LinkOut]
- Beyer T, Bockisch A, Kühl H, et al. Whole-body 18F-FDG PET/CT in the presence of truncation artifacts. J Nucl Med 2006;47:91-9.[LinkOut]
- Goerres GW, Hany TF, Kamel E, et al. Head and neck imaging with PET and PET/CT: artefacts from dental metallic implants. Eur J Nucl Med Mol Imaging 2002;29:367-70.[LinkOut]
- Antoch G, Freudenberg LS, Egelhof T, et al. Focal tracer uptake: a potential artifact in contrast-enhanced dual-modality PET/CT scans. J Nucl Med 2002;43:1339-42.[LinkOut]
- Gould KL, Pan T, Loghin C, et al. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med 2007;48:1112-21.[LinkOut]
- Erdi YE, Nehmeh SA, Pan T, et al. The CT motion quantitation of lung lesions and its impact on PET-measured SUVs. J Nucl Med 2004;45:1287-92.[LinkOut]
- Pevsner A, Nehmeh SA, Humm JL, et al. Effect of motion on tracer activity determination in CT attenuation corrected PET images: a lung phantom study. Med Phys 2005;32:2358-62.[LinkOut]
- Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med 2004;34:122-33.[LinkOut]
- Osman MM, Cohade C, Nakamoto Y, et al. Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. J Nucl Med 2003;44:240-3.[LinkOut]
- Beyer T, Rosenbaum S, Veit P, et al. Respiration artifacts in whole-body (18) F-FDG PET/CT studies with combined PET/CT tomographs employing spiral CT technology with 1 to 16 detector rows. Eur J Nucl Med Mol Imaging 2005;32:1429-39.[LinkOut]
- Goerres GW, Kamel E, Seifert B, et al. Accuracy of image coregistration of pulmonary lesions in patients with non-small cell lung cancer using an integrated PET/CT system. J Nucl Med 2002;43:1469-75.[LinkOut]
- de Juan R, Seifert B, Berthold T, et al. Clinical evaluation of a breathing protocol for PET/CT. Eur Radiol 2004;14:1118-23.[LinkOut]
- Goerres GW, Kamel E, Heidelberg TN, et al. PET-CT image co-registration in the thorax: influence of respiration. Eur J Nucl Med Mol Imaging 2002;29:351-60.[LinkOut]
- Hamill JJ, Bosmans G, Dekker A. Respiratory-gated CT as a tool for the simulation of breathing artifacts in PET and PET/CT. Med Phys 2008;35:576-85.[LinkOut]
- Nehmeh SA, Erdi YE, Meirelles GS, et al. Deep-inspiration breath-hold PET/CT of the thorax. J Nucl Med 2007;48:22-6.[LinkOut]
- Meirelles GS, Erdi YE, Nehmeh SA, et al. Deep-inspiration breathhold PET/CT: clinical findings with a new technique for detection and characterization of thoracic lesions. J Nucl Med 2007;48:712-9.[LinkOut]
- Torizuka T, Tanizaki Y, Kanno T, et al. Single 20-second acquisition of deep-inspiration breath-hold PET/CT: clinical feasibility for lung cancer. J Nucl Med 2009;50:1579-84.[LinkOut]
- Yamaguchi T, Ueda O, Hara H, et al. Usefulness of a breath-holding acquisition method in PET/CT for pulmonary lesions. Ann Nucl Med 2009;23:65-71.[LinkOut]
- Nagamachi S, Wakamatsu H, Kiyohara S, et al. The reproducibility of deep-inspiration breath-hold (18)F-FDG PET/CT technique in diagnosing various cancers affected by respiratory motion. Ann Nucl Med 2010;24:171-8.[LinkOut]
- Lagerwaard FJ, Van Sornsen de Koste JR, Nijssen-Visser MR, et al. Multiple "slow" CT scans for incorporating lung tumor mobility in radiotherapy planning. Int J Radiat Oncol Biol Phys 2001;51:932-7.[LinkOut]
- van Sörnsen de Koste JR, Lagerwaard FJ, Schuchhard-Schipper RH, et al. Dosimetric consequences of tumor mobility in radiotherapy of stage I nonsmall cell lung cancer--an analysis of data generated using 'slow' CT scans. Radiother Oncol 2001;61:93-9.[LinkOut]
- Nye JA, Esteves F, Votaw JR. Minimizing artifacts resulting from respiratory and cardiac motion by optimization of the transmission scan in cardiac PET/CT. Med Phys 2007;34:1901-6.[LinkOut]
- Pan T, Mawlawi O, Nehmeh SA, et al.Attenuation correction of PET images with respiration-averaged CT images in PET/CT. J Nucl Med 2005;46:1481-7.[LinkOut]
- Pan T, Lee TY, Rietzel E, et al. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med Phys 2004;31:333-40.[LinkOut]
- Cook RA, Carnes G, Lee TY, et al. Respiration-averaged CT for attenuation correction in canine cardiac PET/CT. J Nucl Med 2007;48:811-8.[LinkOut]
- Alessio AM, Kohlmyer S, Branch K, et al. Cine CT for attenuation correction in cardiac PET/CT. J Nucl Med 2007;48:794-801.[LinkOut]
- Pan T, Mawlawi O, Luo D, et al. Attenuation correction of PET cardiac data with low-dose average CT in PET/CT. Med Phys 2006;33:3931-8.[LinkOut]
- Huang TC, Mok GS, Wang SJ, et al. Attenuation correction of PET images with interpolated average CT for thoracic tumors. Phys Med Biol 2011;56:2559-67.[LinkOut]
- Guerrero T, Zhang G, Huang TC, et al. Intrathoracic tumour motion estimation from CT imaging using the 3D optical flow method. Phys Med Biol 2004;49:4147-61.[LinkOut]
- Mok GSP, Sun T, Wu T-H, et al. Interpolated average CT for attenuation correction in PET-A simulation study. IEEE Nucl Sci Symp Med Imag Conf Rec, Valencia, Spain, 2011.
- Nehmeh SA, Erdi YE, Ling CC, et al. Effect of respiratory gating on reducing lung motion artifacts in PET imaging of lung cancer. Med Phys 2002;29:366-71.[LinkOut]
- Boucher L, Rodrigue S, Lecomte R, et al. Respiratory gating for 3-dimensional PET of the thorax: feasibility and initial results. J Nucl Med 2004;45:214-9.[LinkOut]
- Nehmeh SA, Erdi YE, Pan T, et al. Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys 2004;31:3179-86.[LinkOut]
- Pönisch F, Richter C, Just U, et al. Attenuation correction of four dimensional (4D) PET using phase-correlated 4D-computed tomography. Phys Med Biol 2008;53:N259-68.
- Wells RG, Ruddy TD, DeKemp RA, et al. Single-phase CT aligned to gated PET for respiratory motion correction in cardiac PET/CT. J Nucl Med 2010;51:1182-90.[LinkOut]
- Nagel CC, Bosmans G, Dekker AL, et al. Phased attenuation correction in respiration correlated computed tomography/positron emitted tomography. Med Phys 2006;33:1840-7.[LinkOut]
- Livieratos L, Stegger L, Bloomfield PM, et al. Rigid-body transformation of list-mode projection data for respiratory motion correction in cardiac PET. Phys Med Biol 2005;50:3313-22.[LinkOut]
- Lamare F, Ledesma Carbayo MJ, Cresson T, et al. List-mode-based reconstruction for respiratory motion correction in PET using non-rigid body transformations. Phys Med Biol 2007;52:5187-204.[LinkOut]
- Thorndyke B, Schreibmann E, Koong A, et al. Reducing respiratory motion artifacts in positron emission tomography through retrospective stacking. Med Phys 2006;33:2632-41.[LinkOut]
- Dawood M, Buther F, Jiang X, et al. Respiratory motion correction in 3-D PET data with advanced optical flow algorithms. IEEE Trans Med Imaging 2008;27:1164-75.[LinkOut]


