Ultrasonographic clues for diagnosis of spina bifida occulta in children
Introduction
Spina bifida occulta (SBO) is an abnormality of the posterior arch formation, and its prevalence is reported to be as high as 22% (1). It is a disorder of spinal development in childhood that will, as it does not improve spontaneously, after posterior element maturation, will persist into adult life. The most common vertebral sites involved are either S1 alone, or S1 and S2 together (2,3). SBO is asymptomatic in the vast majority of patients (2,3). SBO has recently been linked with voiding problems including nocturnal enuresis. Due to a possible association between SBO and spinal cord abnormalities, assessment and radiological evaluation of spinal cord gained importance (2,4).
Anatomical studies have demonstrated that the lumbar sympathetic outflow to lower urinary tract muscles originates from spinal cord segments T10 and L2. Therefore, lumbar SBO might be related with incomplete neurogenesis of the sympathetic nerves. Although SBO occurs in the lower lumbar spines, it can be a marker of these abnormalities (5,6). In recent literature, SBO has been shown not to associated with any detectable structural spinal cord abnormalities through imaging modalities including MRI (3,7,8).
Plain radiographs focusing on L5-S1 segments of the spinal cord are used for the diagnostic study of children with nocturnal enuresis. However, radiation exposure can be harmful especially for children (2,4). We hypothesized that ultrasonographic signs might be useful for detection of SBO in children suffering from nocturnal enuresis. In case such a potential is determined, a safe and practical diagnostic alternative may be used. To the best of our knowledge, the radiological signs described in the current study, have not been identified previously in the medical literature according to PubMed search.
The aim of this cross-sectional study was to find out if spinal ultrasonography might have a predictive potential for detection of SBO in paediatric nocturnal enuresis patients and to test the diagnostic potential of ultrasonographic clues for this purpose.
Methods
Study design
This study was carried out in the radiology department of a tertiary care center after the approval of the local Institutional Review Board and Ethics Committee. Written informed consent was obtained from the parents of all subjects.
Initially, 169 nocturnal enuresis patients referred by the paediatric surgery department of our tertiary center, between January 2014 and May 2014, were enrolled in the study. Diagnosis of SBO has been confirmed by plain radiography in 140 patients. Computerized tomography (CT) scans were obtained from 29 nocturnal enuresis patients for whom gas interposition on plain radiographs did not allow an accurate assessment of spinous processes on L5 and S1 levels. In addition, CT scan had been requested in nine patients for the elimination of urolithiasis at distal ureter and pelvic malignancies.
Fifty patients whose L5-S1 segments could not be evaluated due to gas interposition and 11 patients who were lost to ultrasonography after CT were excluded from the study. Hence, remaining 108 patients were allocated into two groups: Group 1 (n=54, 50%): nocturnal enuresis paediatric patients with SBO; Group 2 (n=54, 50%): nocturnal enuresis paediatric patients without SBO.
Exclusion criteria included physical signs of spina bifida (e.g., lipomas, hemangiomas, hairy patches, asymmetrical gluteal crease), other urological diseases (such as posterior urethral valve, bulbous urethral ring, ureterocele, vesicoureteral reflux), any other neurological abnormalities (else than the spinal cord abnormalities under investigation) or any other associated disorders (myelomeningocele, lipomyelomeningocele, anorectal malformations, etc.).
None of the children had been diagnosed with any known urological or neurological abnormalities previously. All cases underwent a complete physical examination, including inspection of the lumbosacral spine, neurological evaluation of the lower limbs, perianal sensation and tonus of anal sphincter. Plain abdominal radiographs obtained in all patients for the presence or absence of fusion of the posterior elements of the lumbar and/or sacral vertebrae.
Radiological assessment
On plain radiographs of the spine, the presence or absence of fusion of the posterior elements of the lumbar and/or sacral spinous processes was examined to detect SBO.
Ultrasonography was performed by a radiologist using a 7.5 frequency linear probe (Toshiba Aplio 500, Toshiba Medical Systems Corporation, Tokyo, Japan). All images (sagittal, axial) obtained were saved on the computer. The patient was placed in the prone position, and the probe was positioned appropriately for viewing 5th lumbar and 1st sacral vertebral bodies on the sagittal plane using the SI joints as a marker. After observation of the angle formed by these vertebral bodies, spinous process is identified, and assessment is made on the axial sonographic plane. The probe is positioned about 60° caudally to view both the spinous process and laminae of 5th lumbar and 1st sacral vertebra.
Outcome parameters
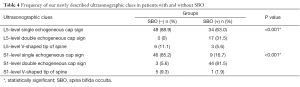
Radiologists who performed spinal and abdominal ultrasonographies were blinded to the radiographs of patients. The ultrasonographic clues (single and double echogeneous cap signs, the V-shaped tip of spine) that we have proposed were investigated in patients with and without SBO (Figures 1-3). Single echogeneous cap sign is “the appearance of an approximately 5 mm echogeneous line confirming the complete fusion of spinous processes and posterior arch in cases without SBO” (Figure 1A). Double echogeneous cap sign is defined as “two posterior laminae looking as two distinct echogeneous foci” and is consistent with a diagnosis of SBO (Figure 3A). V-shaped tip of the spine is defined as “anterior notching seen in tips of posterior laminae due to the partial fusion of posterior spinal arch”. In other words, it reflects the fusion of anterior spinal process, while the posterior section is not fused completely resulting in a V-shaped appearance (Figure 2A). This sign may mimic a complete fusion defect in some cases.
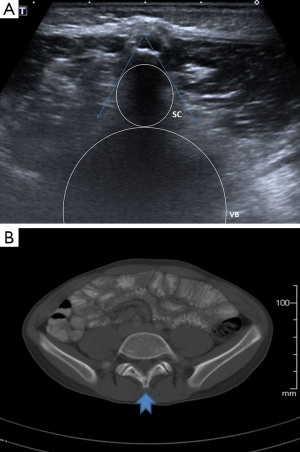
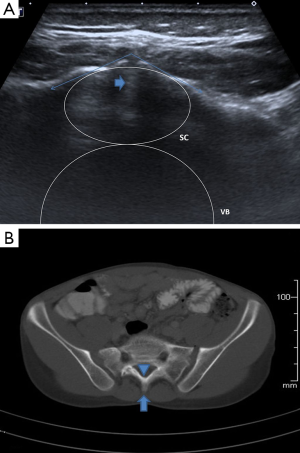
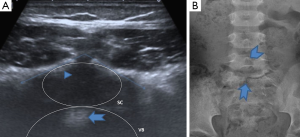
Sensitivity and specificity of these radiological clues were compared to those of plain radiography and CT scans in the diagnosis of SBO.
Statistical analyses
Statistical analysis was performed using the Statistical Package for Social Sciences Software (SPSS 21.0 for Windows, SPSS Inc., Chicago, IL, USA) and Medcalc 9 (Acacialaan 22, B-8400 Ostend, Belgium). The normal distribution of variables was evaluated with Shapiro-Wilk test. Parameters with normal distribution were assessed with parametric methods, whereas nonparametric methods were used for testing variables without normal distribution. Two independent groups are compared via Independent-Samples t-test and Mann-Whitney U test. Categorical variables are analyzed by Pearson Chi-Square test. Performance regarding variability of classification was tested by sensitivity, specificity, positive predictivity, negative predictivity and receiver operating curve (ROC) analysis. Data are displayed as mean and standard deviation or as median and interquartile range. The level of statistical significance was set at P<0.05.
Results
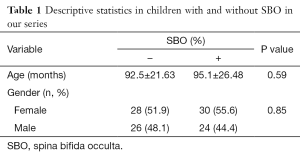
The population consisted of 58 females (53.7%) and 50 males (46.3%). The mean ages for cases with SBO and without SBO were 92.5±21.6 and 95.1±26.5 months, respectively. The incidence of SBO in the whole nocturnal enuresis group enrolled in this study was 50% (54/108). There was no statistically significant difference between SBO and non-SBO groups regarding age (P=0.59) and gender (P=0.85) (Table 1).
Full table
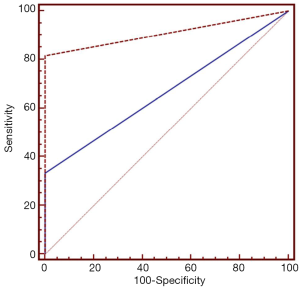
ROC curve analysis of CT and ultrasonographic clues for diagnosis of SBO on S1 level revealed areas under curve for CT and ultrasonographic clues were 0.667±0.053 and 0.907±0.032 (P<0.001) respectively (Figure 4). Tables 2,3 show ROC curve analysis of sensitivity, specificity, and predictive values of gold standard methods (CT scan) for L5 and S1 levels. Frequencies of our newly described ultrasonographic clues in cases with and without SBO are displayed in Table 4. It was seen that single echogeneous cap sign was observed more frequently in patients without SBO, whereas double echogeneous cap sign was consistent with cases diagnosed with SBO (P<0.001).

Full table

Full table
Full table
Discussion
In this study, we aimed to present ultrasonographic clues, which may be useful for diagnosis of SBO in the paediatric population complaining of nocturnal enuresis. We found that single and double echogeneous cap signs and the V-shaped tip of the spine might aid for an easier and cheaper diagnosis of SBO without the risk of radiation exposure after validation and popularization.
Bladder dysfunction is a relatively common complaint in the paediatric population and it can ensource from neuropathic or functional disorders (5,6). Whether SBO and lower urinary tract dysfunction are both consequences of one common defective developmental process is vague (1,7). In summary, there is still considerable controversy on the significance of radiographic SBO in patients with lower urinary tract dysfunction.
The incidence of SBO in the general population is reported as 1−22%, and the incidence of SBO is supposed to be higher in those with incontinence, voiding dysfunction or lower urinary tract abnormality than in the general population (3,8). A significantly higher incidence of SBO had been reported in a patient group suffering from voiding-related symptoms (4,7,8). Gallaway et al. reported that 43% of patients with urinary incontinence also had deformities of vertebral arch (9). Kumar found that 37.5% of children with nocturnal enuresis had SBO, whereas Miyazato et al. reported a much higher SBO rate (69%) for these children (4,5).
SBO has been implicated as a marker for incomplete neurogenesis in the spinal cord (4,8). This theory relies on the assumption that SBO, as a pre-tethered cord, can affect the nerve cells of the lumbar and sacral region (4,7). Samuel and Boddy also established an association between the presence of SBO and lower urinary tract dysfunction. However, they did not come across a link between SBO and specific structural abnormalities in the spinal cord by MRI (2). Nejat et al. also claimed that there was no association between structural dysfunction in the spinal cord and SBO, although they conceded that they were unable to identify the role of SBO in pathophysiology at the cellular level (7).
Plain abdominal radiographs are frequently obtained to screen for lumbosacral abnormalities in this group of patients. Plain radiographs are cheap and practical, but the absence of complex spinal abnormalities on plain radiographs does not preclude the need for more detailed spinal imaging. In addition, owing to the higher radiosensitivity of children than adults, radiation exposure constitutes an important limitation. Magnetic resonance imaging is superior to other modalities regarding evaluation of spinal cord abnormalities. However, it is expensive and has limited availability. The indications for spinal MR imaging in this group remain unclear (8,10,11).
Asakura et al. claimed that it is feasible to accurately diagnose the presence of SBO by the use of ultrasound imaging for neuroaxial anesthesia, thereby paving the way to avoid possible complications (12). They showed that the use of ultrasound imaging efficaciously enables the diagnosis of “hidden” cases of SBO (12). In other words, ultrasound imaging could potentially be of great help as a scanning tool for the diagnosis of SBO before neuroaxial anesthesia is employed. In the present study, we noted that single echogeneous cap sign was useful for ruling out SBO. In other words, this sign was observed in cases devoid of SBO. In contrary, double echogeneous cap sign assisted in ruling in SBO. The third ultrasonographic clue, the V-shaped tip of the spine, was remarkably less sensitive and less specific for the diagnosis of SBO. We suggest that single and double echogeneous cap signs have displayed promising potentials regarding practical and safe diagnostic sonographic clues in paediatric patients suspected for SBO. In the current study, this potential has been documented for lesions involving L5 and S1 levels of vertebrae.
The main limitations of the current study are the relatively small number of patients and lack of documentation of details of history and factors prone to affect the outcome. Since we only involved the paediatric population of Caucasians in our country, the reference values may not be valid for other populations. Furthermore, expression of average values for data derived from both groups may not allow the determination of thresholds and true positive/negative rates. In addition, ultrasonography may possess a limited diagnostic capacity especially in cases with a thick subcutaneous tissue.
Conclusions
To conclude, our results suggest that detection of SBO in the paediatric age group can be facilitated by usage of ultrasonography more efficiently. Our ultrasonographic clues seem to be promising in this purpose. Data obtained from this study can serve as baseline information with which further studies should be conducted for validation and standardization. Thereby, we hope that our findings may be useful in the popularization of ultrasonography as a safe and practical diagnostic modality for SBO in paediatric patients, and unnecessary interventions and measures can be avoided in some cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Institutional Review Board and Ethics Committee (No. 2013/57) and written informed consent was obtained from all patients.
References
- Ritchey ML, Sinha A, DiPietro MA, Huang C, Flood H, Bloom DA. Significance of spina bifida occulta in children with diurnal enuresis. J Urol 1994;152:815-8. [PubMed]
- Samuel M, Boddy SA. Is spina bifida occulta associated with lower urinary tract dysfunction in children? J Urol 2004;171:2664-6. [Crossref] [PubMed]
- Boone D, Parsons D, Lachmann SM, Sherwood T. Spina bifida occulta: lesion or anomaly? Clin Radiol 1985;36:159-61. [Crossref] [PubMed]
- Kumar P, Aneja S, Kumar R, Taluja V. Spina bifida occulta in functional enuresis. Indian J Pediatr 2005;72:223-5. [Crossref] [PubMed]
- Miyazato M, Sugaya K, Nishijima S, Owan T, Ogawa Y. Location of spina bifida occulta and ultrasonographic bladder abnormalities predict the outcome of treatment for primary nocturnal enuresis in children. Int J Urol 2007;14:33-8. [Crossref] [PubMed]
- Kalra V, Palaksha HK. Incidental spina bifida occulta in functional enuresis observed during laser reflexo therapy. J Child Neurol 1999;14:541-3. [Crossref] [PubMed]
- Nejat F, Radmanesh F, Ansari S, Tajik P, Kajbafzadeh A, El Khashab M. Spina bifida occulta: is it a predictor of underlying spinal cord abnormality in patients with lower urinary tract dysfunction? J Neurosurg Pediatr 2008;1:114-7. [Crossref] [PubMed]
- Shin SH, Im YJ, Lee MJ, Lee YS, Choi EK, Han SW. Spina bifida occulta: not to be overlooked in children with nocturnal enuresis. Int J Urol 2013;20:831-5. [Crossref] [PubMed]
- Galloway NT, Tainsh J. Minor defects of the sacrum and neurogenic bladder dysfunction. Br J Urol 1985;57:154-5. [Crossref] [PubMed]
- McGovern M, Mulligan S, Carney O, Wall D, Moylett E. Ultrasound investigation of sacral dimples and other stigmata of spinal dysraphism. Arch Dis Child 2013;98:784-6. [Crossref] [PubMed]
- Pires CR, de Medeiros JM, Araujo Júnior E, Czapkowski A, Zanforlin Filho SM. Occult spinal dysraphism in the presence of rare cutaneous stigma in a neonate: importance of ultrasound and magnetic resonance imaging. Case Rep Med 2013;2013:468376.
- Asakura Y, Kandatsu N, Hashimoto A, Kamiya M, Akashi M, Komatsu T. Ultrasound-guided neuroaxial anesthesia: accurate diagnosis of spina bifida occulta by ultrasonography. J Anesth 2009;23:312-3. [Crossref] [PubMed]


