An unusual cause of back pain in a child: spinal subdural haematoma secondary to intracranial arachnoid cyst haemorrhage
Spinal subdural haematomas are rare entities which can occur secondary to trauma, clotting disorders, vascular malformations or iatrogenic procedures (1). Less commonly, a spinal subdural hematoma can have an intracranial origin, via gravity-dependent migration of blood into the spinal dural sac (2).
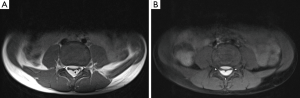
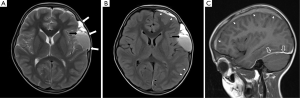
A 7-year-old boy presented to the emergency department with a 1-week gradual onset of worsening lower back pain. There was no history of trauma and he had previously been fit and healthy. On examination, there was no neurological compromise and his vital signs were normal. Blood tests, urinalysis and lumbar spine X-ray were unremarkable. Magnetic resonance imaging (MRI) of his lumbar spine was performed which showed linear T1-hyperintense collections dorsal and ventral to the lower cord and cauda equina, suspicious for spinal subdural haematomas (Figure 1A,B). MRI of the remainder of the neural axis and additional fat-saturated T1 sequences of the lumbar spine were subsequently obtained to confirm the presence of blood, determine their extent and identify a cause. The cervical and thoracic spine scans showed extension of the collections up to the level of C7 (Figure 1C). Axial fat-saturated T1 sequences confirmed the presence of blood within the subdural space (Figure 2). The brain MRI revealed a large arachnoid cyst in the left middle cranial fossa complicated by intracystic and subdural haemorrhage (Figure 3). Further history elicited from the patient was negative for headaches, nausea or previous head injury. A neurosurgical opinion was sought regarding these findings. As there was no midline shift or cauda equina compression on imaging and the patient was neurologically intact, surgical intervention was not deemed necessary at the time. The patient was managed conservatively with analgesia and continued to remain stable clinically. He was discharged 5 days after admission with planned neurosurgical and haematological follow-up.
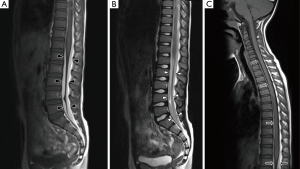
In a large review by Kreppel et al., isolated spinal subdural haematomas were found to comprise only 4% of all spinal haemorrhages (3). This could be because, unlike the cranial meninges, the spinal meninges have no bridging veins between the dura and arachnoid which are vulnerable to tearing injury (4). The vast majority of spinal subdural haematomas are caused by either trauma or bleeding disorders (1). In the absence of these predisposing factors, an intracranial origin of the subdural blood should be suspected.
Differentials for spinal subdural haematoma on MRI include extradural haematoma, epidural lipomatosis and subdural abscess. Subdural haematomas can be differentiated from extradural haematomas on T1-weighted sequences by identifying a thin line of hypointense dura between the subdural collection and hyperintense epidural fat (5) (Figure 2A). Epidural lipomatosis can appear similar to a subacute subdural haematoma, as both are T1-hyperintense. However, on a fat-saturated T1 sequence, epidural lipomatosis would suppress whereas subdural blood would remain hyperintense (Figure 2B). A subdural abscess can resemble a hyperacute subdural haematoma as both would appear T1-isointense and T2-hyperintense. Post-contrast sequences can be helpful in differentiating the two entities. A subdural abscess would demonstrate diffuse, heterogeneous enhancement while a subdural haematoma can be non-enhancing or demonstrate only mild peripheral enhancement.
Arachnoid cysts are benign, congenital, CSF-filled lesions which do not communicate with the ventricular system. They are most commonly found in the middle cranial fossa, anterior to the temporal lobes. Other common locations include the cerebellopontine angle and the suprasellar cistern. Arachnoid cysts are more common in males. They are usually asymptomatic and remain stable in size throughout the individual’s lifespan (6). Intracystic and subdural haemorrhages are rare complications of arachnoid cysts, which can arise spontaneously or following head trauma (7). Fewer than 30 cases of spontaneous intracystic haemorrhage have been reported in the literature, at least six of which occurred in patients under the age of 18 (7-11). Patients with these complications would be expected to present with headache, vomiting or neck stiffness (11,12). However, our patient did not describe any of these symptoms.
On MRI, arachnoid cysts are seen as well-defined, extra-axial lesions with internal signal that follows CSF on all sequences. They do not enhance with contrast. Scalloping of the overlying skull vault and hypoplasia of the adjacent brain parenchyma can be seen, secondary to remodeling by the cyst (Figure 3). Arachnoid cysts suppress completely on fluid-attenuated inversion recovery (FLAIR) and do not restrict on diffusion-weighted imaging—two features which differentiate them from epidermoid cysts (6). Lack of suppression on FLAIR and fluid levels within the cyst are features of intracystic hemorrhage (Figure 3B).
Our patient was managed conservatively because there was no significant mass effect on MRI and no signs of neurological compromise. In a similar case described by Lohani et al., the patient was also managed non-operatively, although oral steroids were given due to clinical evidence of radiculopathy (11). Spontaneous resolution of the intracranial and spinal haematomas was reported in that case and has also been described elsewhere in the literature (11-13). If there is mass effect or neurological deficit, several surgical options are available. Drainage of the intracranial subdural haematoma through a burr hole, leaving the cyst intact, has been advocated as first-line operative management. Other surgical options include craniotomy and cyst resection, endoscopic fenestration and cystoperitoneal shunting (14). Spinal subdural haematomas would be managed with decompressive laminectomy and haematoma evacuation.This case illustrates the importance of imaging the entire neuraxis when a spinal subdural haematoma is seen, to exclude an intracranial origin for the bleed. In children, bleeding from an intracranial arachnoid cyst should be considered as a potential cause for subdural haematomas, even in the absence of a history of trauma or headache.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Treister DS, Kingston SE, Zada G, Singh M, Jones JG, Mills JN, Lerner A, Boyko OB, Law M, Rajamohan A, Shiroishi MS. Concurrent intracranial and spinal subdural hematoma in a teenage athlete: a case report of this rare entity. Case Rep Radiol 2014;2014:143408.
- Bortolotti C, Wang H, Fraser K, Lanzino G. Subacute spinal subdural hematoma after spontaneous resolution of cranial subdural hematoma: causal relationship or coincidence? Case report. J Neurosurg 2004;100:372-4. [PubMed]
- Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003;26:1-49. [Crossref] [PubMed]
- Kanamaru H, Kanamaru K, Araki T, Hamada K. Simultaneous Spinal and Intracranial Chronic Subdural Hematoma Cured by Craniotomy and Laminectomy: A Video Case Report. Case Rep Neurol 2016;8:72-7. [Crossref] [PubMed]
- Boukobza M, Haddar D, Boissonet M, Merland JJ. Spinal subdural haematoma: a study of three cases. Clin Radiol 2001;56:475-80. [Crossref] [PubMed]
- Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology 2006;239:650-64. [Crossref] [PubMed]
- Iaconetta G, Esposito M, Maiuri F, Cappabianca P. Arachnoid cyst with intracystic haemorrhage and subdural haematoma: case report and literature review. Neurol Sci 2006;26:451-5. [Crossref] [PubMed]
- Hong JC, Kim MS, Chang CH, Kim SH. Arachnoid cyst with spontaneous intracystic hemorrhage and chronic subdural hematoma. J Korean Neurosurg Soc 2008;43:54-6. [Crossref] [PubMed]
- Arora R, Puligopu AK, Uppin MS, Purohit AK. Suprasellar Arachnoid Cyst with Spontaneous Intracystic Hemorrhage: A Rare Complication - Role of MR and Illustration of a Case. Pol J Radiol 2014;79:422-5. [Crossref] [PubMed]
- Patel AP, Oliverio PJ, Kurtom KH, Roberti F. Spontaneous Subdural Hematoma and Intracystic Hemorrhage in an Arachnoid Cyst. Radiol Case Rep 2015;4:298. [Crossref] [PubMed]
- Lohani S, Robertson RL, Proctor MR. Ruptured temporal lobe arachnoid cyst presenting with severe back pain. J Neurosurg Pediatr 2013;12:281-3. [Crossref] [PubMed]
- Nagashima H, Tanida A, Hayashi I, Tanishima S, Nanjo Y, Dokai T, Teshima R. Spinal subdural haematoma concurrent with cranial subdural haematoma: Report of two cases and review of literature. Br J Neurosurg 2010;24:537-41. [Crossref] [PubMed]
- Hung KS, Lui CC, Wang CH, Wang CJ, Howng SL. Traumatic spinal subdural hematoma with spontaneous resolution. Spine (Phila Pa 1976) 2002;27:E534-8. [Crossref] [PubMed]
- Kwak YS, Hwang SK, Park SH, Park JY. Chronic subdural hematoma associated with the middle fossa arachnoid cyst: pathogenesis and review of its management. Childs Nerv Syst 2013;29:77-82. [Crossref] [PubMed]


