Towards consistency for magnetic resonance (MR) relaxometry of lumbar intervertebral discs
Lumber disc degeneration is a potential cause of low back pain (LBP). Though disc degeneration itself is not diagnostic for LBP (1-4), in a recent meta-analysis for adults 50 years of age or younger, Brinjikji et al. (5) reported disc degeneration is associated with LBP (OR, 2.24; 95% CI, 1.21–4.15; P=0.01). Many studies on magnetic resonance (MR) relaxometry and disc degeneration have been published (6-8). Novel minimally invasive therapies, such as with injected growth factors or genetic materials, have the potential clinical application to treat pathological disc degeneration (9). MR relaxometry may be useful in clinical trials to evaluate the efficacy of these therapies (9,10).
This issue of QIMS published an interesting paper by Menezes-Reis et al. (11). It is a prospective, cross-sectional and observational study of 90 asymptomatic volunteers at relatively young age 27.1±4.8 years old (range, 20–40 years), and these subjects had low-level physical activity history. Though the T1rho acquisition approach in the study was suboptimal with spin lock frequency of 250 Hz, a few points in their results deserve attention. There was no T2 relaxometry difference between the anterior and posterior annulus fibrosus for their subjects aged 20 to 40 years; however, in the anterior annulus fibrosus T1rho relaxometry values were higher than in the posterior annulus fibrosus. We did additional analysis for the cohorts we previous reported (12). In our study the T1rho of anterior outer annulus fibrosus (AUAF) and posterior outer annulus fibrosus (PUAF) was 53.6±9.6 and 50.7±9.3 msec respectively (P<0.001), and The T2 of AUAF and PUAF was 44.2±9.1 and 36.9±8.6 msec respectively (P<0.001). These results highlight the necessity and importance of analyzing anterior and posterior annulus fibrosus separately, as recently suggested by Ogon et al. (13).
Menezes-Reis et al. (11) noted in their subjects there was a negative correlation between age and disc T2 relaxation time of the whole disc, nucleus pulposus (NP) and posterior annulus fibrosus at all lumbar disc levels. They demonstrated T2 relaxometry detected gradual disc dehydration in the first two decades of adulthood. However, they observed no statistical significant correlation between aging and disc T1rho relaxation both for NP and annulus fibrosus for their subjects (age range, 20–40 years). For L1/2–L4/5 discs, we noted that the age associated reduction of T1rho of NP had a slope of −1.06, while that of T2 had a slope of −1.47, and T2 may be more sensitive at looking at age related relaxometry reduction for NP (14). It should be noted that Menezes-Reis et al.’s study is small in subject number, and for some parameters such as the association between body mass index (BMI), weight and disc relaxometry, the statistical power might not be satisfied.
A number of potential magnetic resonance imaging (MRI) based disc degeneration technique have been published. However, spine specialists are not particularly impressed by the advance on MR relaxometry contributed by MRI community (6,7). Most of the papers demonstrated MR relaxometry confirms the known physiological phenomenon, but does not seem to be useful for diagnostic practice. For translational research aiming for clinical application, I suggest the following points should be considered:
- There is a need to establish age specific and region specific normal ranges of relaxivity values. These normal ranges should be validated cross sequences and cross MRI vendors. With known sensitivity and specificity value, significant deviation from the normal ranges of disc relaxivity may therefore suggest accelerated aging or pathologies. As a way of example, recently Deng et al. (15) and Allkemper et al. (16) reported similar mean value for healthy liver parenchyma of 43.2±2.2 msec (at 3.0 Tesla magnet) and 40.9±2.9 msec (at 1.5 Tesla magnet), respectively;
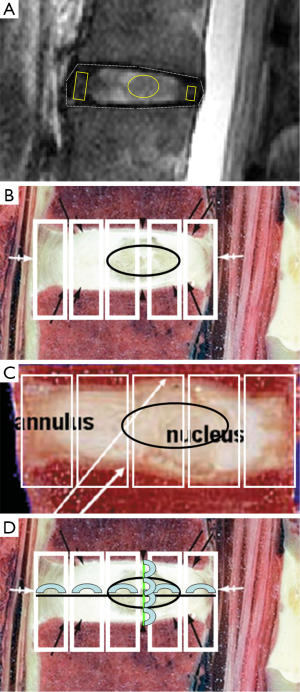
- Currently published approaches for anterior and posterior annulus fibrosus, and NP suffer from poor or sub-optimal measurement reproducibility (17). There is a need to establish standardized and reproducible segmentation method. Probably there is a need of acquiring more than a single central slice in the sagittal plane, such as to include three sagittal slices for relaxometry mapping. Segmentation should be based on clear anatomical landmarks, and together with spine size proportionally determined regions of interests (ROI). This is important as accurate segmentation of components is difficult for degenerated discs when the demarcation between annulus fibrosus and NP become fussy, and together with disc space narrowing, portions of NP might protrude into annulus fibrosus (18). A tentative standardized segmentation approach is suggested in Figure 1; Due to the differences in the extracellular matrix composition, the annulus fibrosus is divided into an inner annulus fibrosus and outer annulus fibrosus. Till now, in many papers the inner annulus fibrosus is often included as part of NP, instead of annulus fibrosus. The implication of this may need to be further clarified (21). Segmentation of inner annulus fibrosus is certainly difficult for degenerated discs (17,22);
- More efforts should be taken to compare and validate novel MR techniques, and to find the complementary roles of each technique (6,7,23). Ideally, MR readouts should be finally validated against clinical readouts (clinical endpoints). MR readouts that characterize intra-discal inflammatory changes remain to be further established (9). Many exploratory MR technically driven studies are being published. However, if a publication of small pilot study was not eventually followed-up by a larger confirmative study (studies); the result of the small study is probably not reproducible. For the later case, an open science approach to synthesize data from multiple centers may produce more reliable conclusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet 2009;373:463-72. [Crossref] [PubMed]
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72:403-8. [PubMed]
- Peterson CK, Bolton JE, Wood AR. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine (Phila Pa 1976) 2000;25:218-23. [Crossref] [PubMed]
- Kovacs FM, Arana E, Royuela A, Estremera A, Amengual G, Asenjo B, Sarasíbar H, Galarraga I, Alonso A, Casillas C, Muriel A, Martínez C, Abraira V. Disc degeneration and chronic low back pain: an association which becomes nonsignificant when endplate changes and disc contour are taken into account. Neuroradiology 2014;56:25-33. [Crossref] [PubMed]
- Brinjikji W, Diehn FE, Jarvik JG, Carr CM, Kallmes DF, Murad MH, Luetmer PH. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol 2015;36:2394-9. [Crossref] [PubMed]
- Belavý DL, Albracht K, Bruggemann GP, Vergroesen PP, van Dieën JH. Can Exercise Positively Influence the Intervertebral Disc? Sports Med 2016;46:473-85. [Crossref] [PubMed]
- Brayda-Bruno M, Tibiletti M, Ito K, Fairbank J, Galbusera F, Zerbi A, Roberts S, Wachtel E, Merkher Y, Sivan SS. Advances in the diagnosis of degenerated lumbar discs and their possible clinical application. Eur Spine J 2014;23 Suppl 3:S315-23. [Crossref] [PubMed]
- Wáng YX. On Magnetic Resonance Imaging of Intervertebral Disc Aging. Sports Med 2016. Epub ahead of print. [Crossref] [PubMed]
- Lotz JC, Haughton V, Boden SD, An HS, Kang JD, Masuda K, Freemont A, Berven S, Sengupta DK, Tanenbaum L, Maurer P, Ranganathan A, Alavi A, Marinelli NL. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology 2012;264:6-19. [Crossref] [PubMed]
- Wang YX. Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin Radiol 2005;60:1051-7. [Crossref] [PubMed]
- Menezes-Reis R, Salmon CE, Bonugli GP, Mazoroski D, Tamashiro MH, Savarese LG, Nogueira-Barbosa MH. Lumbar intervertebral discs T2 relaxometry and T1ρ relaxometry correlation with age in asymptomatic young adults. Quant Imaging Med Surg 2016;6:402-12.
- Wang YX, Zhao F, Griffith JF, Mok GS, Leung JC, Ahuja AT, Yuan J. T1rho and T2 relaxation times for lumbar disc degeneration: an in vivo comparative study at 3.0-Tesla MRI. Eur Radiol 2013;23:228-34. [Crossref] [PubMed]
- Ogon I, Takebayashi T, Takashima H, Tanimoto K, Ida K, Yoshimoto M, Fujiwara H, Kubo T, Yamashita T. Analysis of chronic low back pain with magnetic resonance imaging T2 mapping of lumbar intervertebral disc. J Orthop Sci 2015;20:295-301. [Crossref] [PubMed]
- Wang YX, Griffith JF, Leung JC, Yuan J. Age related reduction of T1rho and T2 magnetic resonance relaxation times of lumbar intervertebral disc. Quant Imaging Med Surg 2014;4:259-64. [PubMed]
- Deng M, Zhao F, Yuan J, Ahuja AT, Wang YX. Liver T1ρ MRI measurement in healthy human subjects at 3 T: a preliminary study with a two-dimensional fast-field echo sequence. Br J Radiol 2012;85:e590-5. [Crossref] [PubMed]
- Allkemper T, Sagmeister F, Cicinnati V, Beckebaum S, Kooijman H, Kanthak C, Stehling C, Heindel W. Evaluation of fibrotic liver disease with whole-liver T1ρ MR imaging: a feasibility study at 1.5 T. Radiology 2014;271:408-15. [Crossref] [PubMed]
- Mok GS, Zhang D, Chen SZ, Yuan J, Griffith JF, Wang YX. Comparison of three approaches for defining nucleus pulposus and annulus fibrosus on sagittal magnetic resonance images of the lumbar spine. J Orthop Translat 2016;6:34-41. [Crossref]
- Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J 2005;14:27-35. [Crossref] [PubMed]
- Haughton VM. Chapter 6: Age-Related Changes in the Spine. In: Naidich TP, Castillo M, Cha S, et al. eds. Imaging of the Spine. Philadelphia: Elsevier, 2010:147-60.
- Urban JP, Winlove CP. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging 2007;25:419-32. [Crossref] [PubMed]
- Schiebler ML, Grenier N, Fallon M, Camerino V, Zlatkin M, Kressel HY. Normal and degenerated intervertebral disk: in vivo and in vitro MR imaging with histopathologic correlation. AJR Am J Roentgenol 1991;157:93-7. [Crossref] [PubMed]
- Wáng YX, Zhang Q, Li X, Chen W, Ahuja A, Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg 2015;5:858-85. [PubMed]
- Wáng YX, Griffith JF. Biomedical imaging in translational orthopaedic research. J Orthop Translat 2015;3:157-9. [Crossref]


