Effects of magnetic field strength and b value on the sensitivity and specificity of quantitative breast diffusion-weighted MRI
Introduction
Diffusion-weighted imaging (DWI) is sensitive to the Brownian motion of water molecules and provides a quantitative measure of the microscopic cellular environment without a need for an exogenous contrast agent. In oncologic imaging, restricted diffusion has been found to correlate with increased cellularity in the tumor tissue (1-3). The degree of sensitivity of DWI images to water diffusion is controlled by a user-selectable parameter known as the b value, which is expressed in unit of s/mm2. Because tissues with high T2 may also appear hyperintense on DWI images, a quantitative map of apparent diffusion coefficient (ADC) can be calculated from two or more DWI images of different b values to remove the T2 “shine-through” effects (4). The pixel value of a quantitative ADC map is a measure of the average squared displacement of a water molecule within the pixel through diffusion per unit time and is expressed in unit of mm2/s (5).
In an ideal case of a single isotropic diffusion, the signal intensity of the DWI images decays exponentially as a function of the b value, and ADC can be determined with two DWI images acquired at any two different b values. However, DWI images generated at low b values may be affected by non-diffusion phenomena, such as microcapillary perfusion or intravoxel incoherent motion (IVIM) of water molecules in the circulating blood (6,7). Although a DWI image with a larger b value is more sensitive to water diffusion and may provide a better contrast between malignant and benign tissue than a DWI image with a lower b value, a high b value also results in an overall decreased signal-to-noise ratio (SNR) and may render the calculation of ADC inaccurate and unreliable (5,8).
There is currently no consensus in regard to which b value would yield the best diagnostic performance for the detection and characterization of breast malignancy (9). While some investigators have suggested that higher quality DWI and ADC maps are achieved with a b value of 850 s/mm2 (10), others have concluded that high b values (e.g., 1,500) result in better image contrast between various malignant breast lesions, ductal carcinoma in situ (DCIS), and benign lesions (11,12). Previous reports also suggest a higher lesion conspicuity for breast MRI using a 3.0-T magnetic field strength (B0) when compared to using a 1.5-T field strength (13). However, it is not clear whether 3.0 T would have a significant effect on the diagnostic performance of DWI in differentiating malignant from benign breast lesions.
We undertook the study to determine the optimal thresholds of ADC in breast DWI and to evaluate the effect of the magnetic field strength and b value of breast DWI images on the sensitivity and specificity to differentiate malignant from benign breast lesions.
Methods
This study was conducted as a clinical performance quality improvement (PQI) project to optimize the image quality of our institution’s routine breast MRI. Approval for retrospective chart review was obtained from the Institutional Review Board (IRB) and HIPPA compliance was strictly adhered to (No. DR11-0048). Two DWI sequences using b values of (0, 1,000) and (0, 1,500) s/mm2 were added to all clinical breast MRI studies that included conventional fat-suppressed T2-weighted sequence and dynamic contrast-enhanced T1-weighted sequences for the purpose of determining the optimal protocol for standard of care. The patients were randomly assigned to one of the four MRI scanners (GE Healthcare Technologies, Waukesha, WI, USA) that were in use at our institution for clinical breast MRI studies based on which scanner was available at the time of the examination. Two of the four scanners had a field strength of 1.5 Tesla and the other two scanners were 3.0 Tesla. All the scanners were equipped with the twin-resonance-module (TRM) gradients and were operating at the HDxt 16.0 software platform. All studies used an eight-channel high-density dedicated bilateral breast radiofrequency (RF) coil (GE Healthcare Technologies, Waukesha, WI, USA). Both DWI sequences were based on single shot echo-planar imaging (EPI), with a parallel imaging acceleration factor of two along the phase encode direction (left/right). The scan parameters are listed as below and were kept identical for the two sequences and at the two field strengths except as noted: TR =4,500–5,000 ms, TE =71–73 ms (when b value =1,000 s/mm2) and TE =78–80 ms (when b value =1,500 s/mm2), receiver bandwidth =±250 kHz, FOV =28–36 cm, slice thickness/gap =4–5 mm/0; acquisition matrix =160×224 at 1.5 T and acquisition matrix =160×256 at 3.0 T, number of signal average (NEX) =4, and the total acquisition time was around 4 minutes for each sequence depending on the required spatial coverage. The MRI studies were reviewed by two dedicated breast radiologists with breast MRI experience of 12 and 11 years, respectively. Inclusion criteria for the study were technically acceptable DWI image quality, availability of ADC maps, and availability of histopathology from MRI-guided or second-look ultrasound-guided biopsy when a focal lesion was seen. In patients with no focally enhancing lesions, a minimum of 2 years of clinical and imaging follow-up was available. Exclusion criteria were significant artifacts (e.g., geometric distortion, poor fat suppression) limiting lesion evaluation on DWI images (n=25, 13%), bilateral transverse rectus abdominis myocutaneous flap reconstruction (n=4, 2%), and bilateral breast cancer (n=1, 0.5%) with a lack of normal breast tissue as reference.
The studies meeting the inclusion criteria were divided into two groups based on the presence or absence of a focally enhancing lesion on the dynamic contrast enhanced images. If the breast MRI demonstrated an enhancing lesion, the radiologists visually evaluated the ADC and DWI images of the lesion, outlined a round region of interest with an area of ~1 cm2 over the lesion, and measured the mean ADC values (ADCmean) of the lesion on both ADC maps that were generated from the two DWI sequences using b value = (0, 1,000) or b value = (0, 1,500) s/mm2, respectively. In the same patients, a round region of interest was similarly outlined over normal glandular breast tissue in a corresponding area of the contralateral breast, and the ADCmean for the normal tissue region was also recorded. If the breast tissue in the breast MRI images was considered normal with no abnormal enhancing lesion on the dynamic enhanced images, a similar approach was taken: a round region of interest of approximately 1 cm2 was outlined over an area representing normal glandular breast tissue, and the ADCmean values from both ADC maps were recorded.
Measured ADC values were stratified by malignant (with a biopsy-proven malignancy), benign (with a biopsy-proven benign lesion) histopathology, or normal breast tissue. For statistical analysis, we considered the healthy contralateral breasts of patients who had unilateral breast lesions as the normal breast tissue and included the corresponding ADCmean values in the pool of normal breast measurements. Paired and unpaired t-tests and analysis of variance (ANOVA) were performed to compare ADCmean values. Youden’s J statistic was used to calculate the optimal ADC threshold separately for the two DWI sequences in terms of diagnostic accuracy (14). McNemar’s test was used to compare the diagnostic accuracy of the two DWI sequences with the optimal ADC thresholds. A generalized estimating equations (GEE) model using the logit link was used to estimate the probability of a malignant lesion from a benign lesion or normal breast tissue as a function of B0 and the b values (1,000 and 1,500 s/mm2) while accounting for the repeated lesion correlation observed for repeated b values within a patient. Lesions were treated independently, and the model was used to account for dual imaging by different b values. All statistical analyses were performed using the SAS 9.3 software package for Windows.
Results
Eighty-six (68%) of the included MRI examinations were performed on 1.5 T scanners, and 40 (32%) were performed on 3.0 T scanners (Table 1). The median patient age was 47 years (range, 32–81 years). Eighteen (14%) patients had benign lesions [median size (range), 1.5 (0.6–2.7) cm], 33 (26%) patients had biopsy-proven malignant lesions [median size (range), 2.75 (0.5–14.0) cm], and 75 (60%) patients had normal breast MRI results (Table 1). Since we also recorded the contralateral ADCmean measurement from the normal-appearing breast in patients with benign (n=18) or malignant (n=33) lesions, we had a total of 18 benign measurements, 33 malignant measurements, and 126 normal breast tissue measurements for the statistical analyses.
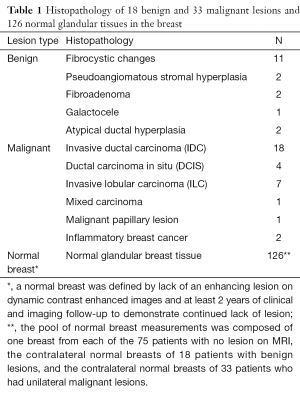
Full table
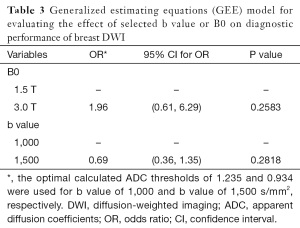
Regardless of B0 and the type of lesions, the value of ADCmean was significantly lower when a b value of 1,500 s/mm2 was used when compared to when a b value of 1,000 s/mm2 was used (Table 2, Figure 1). For malignant lesions, no significant difference was observed in the ADCmean values by the lesion type (ANOVA P>0.05, Figure 2). When the area under the receiver operating characteristic (ROC) curve was maximized, the optimal ADCmean threshold for the b value of 1,000 s/mm2 was 1.235×10–3 mm2/s (sensitivity =93.9, specificity =91.7). The area under the ROC curve was 0.95 (Delong’s 95% confidence interval: 0.88–1.00). Similarly, the optimal ADCmean threshold for the b value of 1,500 s/mm2 was 0.934×10–3 mm2/s (sensitivity =81.8, specificity =97.2). The area under the ROC curve was 0.93 [Delong’s 95% confidence interval (CI): 0.87–0.99]. Using these optimal thresholds, a GEE model was used to evaluate the effects of the selected b value or B0 on the diagnostic performance of DWI (Table 3). The calculated odds ratio were 1.96 for B0 of 1.5 versus 3.0 Tesla (P value =0.26) and 0.69 for the b value of 1,000 versus 1,500 s/mm2 (P value =0.28). The GEE analysis did not show any statistically significant difference in diagnostic performance among the different combinations of the b values (1,000 or 1,500 s/mm2) and magnetic field strength (B0 =1.5 or 3.0 T). The performance of using the optimal ADCmean to differentiate benign from malignant lesions had high diagnostic accuracies ranging from 90% to 96% for the different combinations of B0 and b values (Table 4). The highest diagnostic accuracy of 96% was achieved for the b value of 1,000 s/mm2 at 3.0 T.
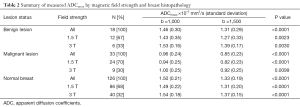
Full table
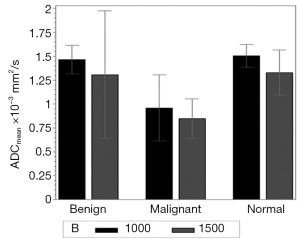
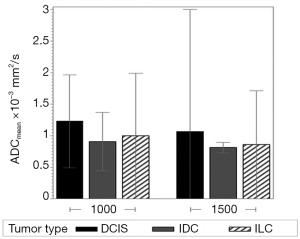
Full table

Full table
Discussion
Using DWI images acquired at two different magnetic field strengths and at two different b values from the same group of patients in the same imaging session, we determined the optimal cutoff ADC values and compared the effect of the b value and B0 on the diagnostic performance of DWI in differentiating benign from malignant breast lesions. Our results showed significantly lower ADCmean values when using b =1,500 s/mm2 compared to when using b =1,000 s/mm2 in all measured tissues similar to a previous report (12). Even for tissues with a mono-exponential dependence on diffusion, the ADC value measured using only two b values is known to be affected by the baseline SNR, the true tissue ADC, and the selected high b values (5). The increased noise floor in the DWI image at high b values can lead to a systematic bias in estimating the signal reduction due to true diffusion and therefore result in a lower measured ADC. The fact that significantly lower ADCmean values were found in the same patients in our study when using b =1,500 s/mm2 compared to when using b =1,000 s/mm2 and that reduced SNR was consistently noted in images at the higher b value (Figure 3) indicates that the measurement of ADC in breast MRI was becoming noise-limited at b =1,500 s/mm2 for the scan protocols we used. Separately, we found that the optimal ADC threshold for differentiating a malignant from a benign lesion or normal breast tissue was lower for the DWI images with higher b values (1.235×10–3 mm2/s for b value =1,000 s/mm2 and 0.934×10–3 mm2/s for b value =1,500 s/mm2). Using the optimal ADC threshold for our b values, we found no significant difference between the selected b values or B0 for the diagnostic accuracy of using the ADCmean to differentiate malignant from benign or normal tissues. Despite a consistent trade-off between sensitivity and specificity when using b value =1,500 s/mm2, the overall diagnostic accuracy remained unchanged when compared to using b value =1,000 s/mm2. The highest diagnostic accuracy of 96% was achieved when using b value =1,000 s/mm2 at an ADC threshold of 1.235×10–3 mm2/s; similar diagnostic accuracy could be achieved when using b value =1,500 s/mm2 at a threshold of 0.934×10–3 mm2/s although the sensitivity became significantly worse.

DWI has been found useful in differentiating benign from malignant breast lesions (6,9). The contrast and signal intensity of a DWI image are dependent on the selected b value. However, the b value that yields the best breast lesion differentiation is still under investigation. In addition, there is limited information on the effect of B0 on the diagnostic accuracy of ADC maps at different ADCmean thresholds. Bogner et al. studied the impact of different b values from 250 to 1,000 s/mm2 and found that breast DWI could differentiate malignant from benign lesions with high diagnostic accuracy of 95% using a b value of 850 s/mm2 and an ADC threshold of 1.25×10–3 mm2/s (10). They reported in the same study that the ADC differentiation and mean contrast-to-noise ratio of DWI for benign and malignant lesions reached a maximum at a b value of 850 s/mm2. Although similar conclusions were reported by others (6,15-17), Woodhams et al. demonstrated that a larger b value of 1,500 s/mm2 may result in a better contrast ratio between invasive tumors, DCIS, and benign lesions (12). Yet, the studies by Pereira et al. (16) and by Chen et al. (17) both reported no statistically significant differences between the ADC values from the different combinations of b values. In a recent meta-analysis of the data from the literature search, Dorrius et al. (18) found that the median ADC values of the different breast tissue types were significantly higher for b values ≤600 s/mm2 than for b values of ≥600 s/mm2. To our knowledge, no study has investigated the effect of B0 on the breast ADC measurements and on differentiating benign from malignant breast lesions.
Our study has several limitations. First, each patient should ideally have been examined on both 1.5 and 3.0 T while keeping everything else the same to better evaluate the effect of B0 on the measured ADC values. However, such an approach is not practical when evaluating patients undergoing actual clinical imaging. Second, a region of interest was manually outlined over the lesion and excluding the T2-hyperintense areas whenever possible. Although the measurement was performed by two experienced breast radiologists, subjective variability could not be completely removed in the resulting ADC measurement, especially for small DCIS lesions, which may have contributed to the relatively large error bars in Figure 2. Finally, the number of patients in each group was limited, with relatively low statistical power in our analyses (12% for the effect of B0 and 9% for the effect of b values).
The calculation of ADC in our study relied on using DWI images with a b value of 0 in all cases. The signal intensity of low b value DWI images may, in general, be affected by the blood vessel microperfusion or IVIM contributions. While it is unclear how significant this effect was for the breast lesions we studied, Baron et al. (19) reported that microperfusion is minimal and does not affect ADC values in breast fibroglandular tissues. At the time of our image acquisition, the DWI software on our scanners did not offer the flexibility of selecting multiple non-zero b values. Future studies that include a small non-zero b value (e.g., 100 s/mm2) and thus help minimize the IVIM contribution may be performed to exclude the potential complication.
In summary, we examined the diagnostic performance and the effect of B0 and b values of DWI for differentiating benign from malignant breast lesions by acquiring and analyzing breast DWI images at the b values of 1,000 and 1,500 s/mm2 and at B0 of 1.5 and 3.0 T. With the optimal ADC thresholds, our results show that a very high diagnostic accuracy of 96% (and sensitivity of 89% and specificity of 98%) can be achieved by DWI for breast lesion differentiation. Our results also show that neither b value nor B0 is a statistically significant factor for its diagnostic performance, although DWI images at a b value of 1,000 s/mm2 have better SNR and may provide more accurate and more reliable measurement of ADC when compared to the images acquired at a b value of 1,500 s/mm2.
Acknowledgements
This work was supported in part by the Cancer Center Support Grant (NCI Grant).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval for retrospective chart review was obtained from the institutional review board (IRB) and HIPPA compliance was strictly adhered to (No. DR11-0048).
References
- Koh DM, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol 2006;79:633-5. [Crossref] [PubMed]
- Brandão AC, Lehman CD, Partridge SC. Breast magnetic resonance imaging: diffusion-weighted imaging. Magn Reson Imaging Clin N Am 2013;21:321-36. [Crossref] [PubMed]
- Inoue K, Kozawa E, Mizukoshi W, Tanaka J, Saeki T, Sakurai T, Kimura F. Usefulness of diffusion-weighted imaging of breast tumors: quantitative and visual assessment. Jpn J Radiol 2011;29:429-36. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497-505. [Crossref] [PubMed]
- Koh DM, Thoeny HC. editors. Diffusion-weighted MR imaging: applications in the body. Wurzburg, Germany: Springer-Verlag, 2010.
- Iacconi C. Diffusion and perfusion of the breast. Eur J Radiol 2010;76:386-90. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-7. [Crossref] [PubMed]
- Dietrich O, Heiland S, Sartor K. Noise correction for the exact determination of apparent diffusion coefficients at low SNR. Magn Reson Med 2001;45:448-53. [Crossref] [PubMed]
- Thomassin-Naggara I, De Bazelaire C, Chopier J, Bazot M, Marsault C, Trop I. Diffusion-weighted MR imaging of the breast: advantages and pitfalls. Eur J Radiol 2013;82:435-43. [Crossref] [PubMed]
- Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, Moser E, Helbich TH, Trattnig S. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology 2009;253:341-51. [Crossref] [PubMed]
- Peled S, Whalen S, Jolesz FA, Golby AJ. High b-value apparent diffusion-weighted images from CURVE-ball DTI. J Magn Reson Imaging 2009;30:243-8. [Crossref] [PubMed]
- Woodhams R, Inoue Y, Ramadan S, Hata H, Ozaki M. Diffusion-weighted imaging of the breast: comparison of b-values 1000 s/mm2 and 1500 s/mm2. Magn Reson Med Sci 2013;12:229-34. [Crossref] [PubMed]
- Matsuoka A, Minato M, Harada M, Kubo H, Bandou Y, Tangoku A, Nakano K, Nishitani H. Comparison of 3.0-and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiat Med 2008;26:15-20. [Crossref] [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Janka R, Hammon M, Geppert C, Nothhelfer A, Uder M, Wenkel E. Diffusion-weighted MR imaging of benign and malignant breast lesions before and after contrast enhancement. Rofo 2014;186:130-5. [PubMed]
- Pereira FP, Martins G, Figueiredo E, Domingues MN, Domingues RC, da Fonseca LM, Gasparetto EL. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol 2009;193:1030-5. [Crossref] [PubMed]
- Chen X, He XJ, Jin R, Guo YM, Zhao X, Kang HF, Mo LP, Wu Q. Conspicuity of breast lesions at different b values on diffusion-weighted imaging. BMC Cancer 2012;12:334. [Crossref] [PubMed]
- Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 2014;24:2835-47. [Crossref] [PubMed]
- Baron P, Dorrius MD, Kappert P, Oudkerk M, Sijens PE. Diffusion-weighted imaging of normal fibroglandular breast tissue: influence of microperfusion and fat suppression technique on the apparent diffusion coefficient. NMR Biomed 2010;23:399-405. [PubMed]


