A large heterotopic ossification in a 25 years old laparotomy scar
Heterotopic ossification (HO) is a benign condition when bone develops in tissues that do not usually ossify. The etiology is not fully understood but the condition can appear in variable locations, sizes and morphology in the body. We describe a large HO in a scar, discovered 25 years after a laparotomy of the upper abdomen. The calcification led to mechanical complaints and resection gave resolution of symptoms. HOs can be incidental findings on plain radiographs. CT-scan can show typical mature peripheral mineralization with central lucency. If symptomatic, resection must be performed after maturation. Maturation can be examined with activity-oriented imaging as 3-phase bone scan or SPECT-CT.
A 53-year-old man presented to the outpatient clinic with complaints of a solid mass in his upper abdomen. His medical history was significant for a post-traumatic laparotomy, 25 years prior. It was unclear what kind of procedure had taken place or if this was only an explorative laparotomy. In recent months, he deliberately lost 10 kg weight. He noticed mechanical obstruction of movement and pain in his upper abdomen while bending forward. Physical examination revealed a large, solid, longitudinal structure, in line and directly under the scar of the upper abdomen laparotomy. It was easily palpated and the caudal margin could be held by the examining physician just above the umbilicus. During examination it appeared to move and/or articulate just below the xyphoid process.
A lateral view plain radiograph of the abdomen showed a large calcification in the anterior abdominal wall (Figure 1). A CT-scan was performed to investigate size, shape and relation to the surrounding structures (Figure 2).

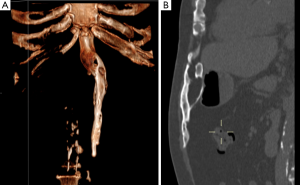
Because of the patient’s request and his mechanical complaints, a surgical resection of this calcification was performed. The old scar was used, a 25-cm median laparotomy from the xyphoid process to the umbilicus. Directly under the fascia of the anterior abdominal wall, a smooth surfaced, irregularly shaped bony calcification of approximately 3 cm by 18 cm size was found (Figure 3).
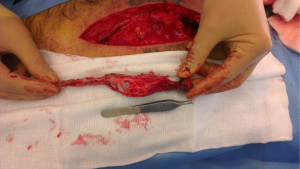
It could be removed easily, besides the cranial attachment to the xyphoid process where connection resembling a pseudo-articulation had been formed. Resection was followed by primary closure. Histopathology of the lesion revealed mature lamellar bone, with a cortical boundary and bone marrow in which the formation of all hematopoietic cell ranges could be seen (Figure 4). The postoperative recovery was uncomplicated. The patient was satisfied with the result of the procedure.

Heterotopic ossification (HO) is a benign condition when bone develops in tissues that do not usually ossify. When this occurs in a muscle, the condition is also known as myositis ossificans. Askanazy first reported the specific localization of HO in an abdominal scar as far back as 1901.
The formation of HO can be categorized in three possible etiologies: traumatic, neurogenic or genetic abnormalities (1). Traumatic HO can appear after fractures, dislocations, burns and after surgical procedures. The latter is most commonly seen around the hip after osteosynthesis of a hip fracture or after hip arthroplasty (2). Neurogenic HO can appear after variable neurological conditions such as spinal injury, head injury or meningitis. The hip and elbow are frequently involved (3) (Garland 1980). Furthermore, HO can occur in the setting of a genetic syndrome, such as progressive osseous heteroplasia or fibrodysplasia ossificans progressiva (4,5). The pathogenesis is not fully understood. It is believed that it is the result of inappropriate differentiation of pluripotent stem cells into osteoblastic stem cells (6). Recent studies support a critical role of bone morphogenetic proteins (BMP’s) in the pathway leading to the formation of HO (7).
Specifically at the formation of HO in abdominal scars, there is a 10:1 ratio of men vs women (8), mainly in vertical scars (9,10). Some authors suggest “seeding” due to damage to the xyphoid process (1). But it is not very likely that this is a necessary condition for the formation of HO, because HO is also found in transverse or lower incisions. Although several cases are reported in the literature, the incidence appears to be low when taking into account the amount of laparotomies that are performed (11). On the other hand, an imaging study of Kim showed HO in surgical incisions of the abdomen in 25% of the patients. This was a consecutive group of patients who underwent both a laparotomy and a postoperative CT-scan at a mean follow up of 378 days. However, the mean craniocaudal length was only 2.3 cm, and it was not reported whether the HOs were symptomatic (10).
HO is frequently asymptomatic. But it can lead to decreased range of motion at a nearby joint, and ankylosis may occur in severe cases (1). It is uncertain when HO in a laparotomy scar leads to symptoms. It seems obvious that mechanical complaints while bending forward are directly related to the size of the HO. As the study of Kim showed an incidence of 25%, we suggest that small HOs in laparotomy scars are frequently asymptomatic (10).
HO is typically an incidental finding on a plain radiograph. Detection of HO in clinically suspect cases is generally performed using either MRI or nuclear imaging. Since these methods can reveal soft tissue reaction and cellular activity as opposed to the calcification seen in late stage HO seen on radiographs. On three-phase bone scan progression can be seen from active phase (positive on all three phases of bone scan) to stabilization where delayed phase bone shows less activity. At maturation the lesion matches normal bone activity. On CT-scan HO shows typical mature peripheral mineralization with central lucency.
The majority of patients with HO are asymptomatic and don’t need any treatment. For the prevention of HO, nonsteroidal anti-inflammatory drugs (e.g., indomethacin) and radiation therapy are used (12). However, when symptoms of HO already have been developed, the only possible treatment is surgical excision. Depending on risk factors, surgical excision can be followed by prophylactic treatment with nonsteroidal anti-inflammatory drugs or radiation therapy.
The optimal timing of surgery is after maturation of the HO, because this decreases the risk of recurrence. This can be evaluated best with activity-oriented imaging such as 3-phase bone scan or SPECT-CT. A 12–18 months delay after the occurrence of HO is suggested (13).
The differential diagnosis of this HO includes a parosteal osteosarcoma and chondrosarcoma, which both are malignant bone forming tumors that must be ruled out. Unlike the HO presented in this case, osteosarcomas are ill defined lesions, with typically a ‘moth eaten pattern’ on a plain radiograph, and can involve the periosteum and adjacent soft tissues (14). The subtype parosteal osteosarcoma can present as an ossified, smooth, lobulated mass which attaches to the underlying bone via a broad pedicle (15). Besides that osteosarcoma differs in the radiological characteristics, histologically an osteosarcoma shows another pattern than HO. Where as HO shows mostly ossification in the periphery, osteosarcomas mostly show a dense ossified centre without the peripheral ossifications. The main difference between HO and malignant bone tumors is that in HO the proliferation of bone tissue is growing from the periphery, in contrast to osteosarcomas from which the tumor will grow from the centre (16). This causes the radiolucent centre in HO, which is not seen in osteosarcomas.
Chondrosarcomas are a heterogeneous group, varying in aggressiveness, typically expansive, mixed lytic and sclerotic with ring like calcifications representing chondroid matrix (17). These lesions clearly need a different work up and treatment. Another diagnostic consideration is an osteochondroma of the xyphoid process. Osteochondromas are common benign bone tumors, and asymptomatic lesions do not need treatment. However, when symptomatic, a resection including the xyphoid process is recommended (18).
Although HOs can be more common than previously suggested, only symptomatic patients need to be treated (11). This case report is illustrative for HOs after laparotomy because it concerns a male patient with an upper midline incision and the location of the ossification in proximity to the xyphoid process. It is an exceptional case because of the size of the HO (18 cm craniocaudal length) and because it became symptomatic after 25 years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys 2006;65:1289-99. [Crossref] [PubMed]
- Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res 1991.13-29. [PubMed]
- Garland DE, Blum CE, Waters RL. Periarticular heterotopic ossification in head-injured adults. Incidence and location. J Bone Joint Surg Am 1980;62:1143-6. [PubMed]
- Cohen RB, Hahn GV, Tabas JA, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty-four patients. J Bone Joint Surg Am 1993;75:215-9. [PubMed]
- Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med 2002;346:99-106. [Crossref] [PubMed]
- Naraghi FF, DeCoster TA, Moneim MS, Miller RA, Rivero D. Heterotopic ossification. Orthopedics 1996;19:145-51. [PubMed]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 2008;14:1363-9. [Crossref] [PubMed]
- Rosa M, Mohammadi A. Myositis ossificans traumatica of the abdominal wall. Can J Surg 2009;52:E33-4. [PubMed]
- Reardon MJ, Tillou A, Mody DR, Reardon PR. Heterotopic calcification in abdominal wounds. Am J Surg 1997;173:145-7. [Crossref] [PubMed]
- Kim J, Kim Y, Jeong WK, Song SY, Cho OK. Heterotopic ossification developing in surgical incisions of the abdomen: analysis of its incidence and possible factors associated with its development. J Comput Assist Tomogr 2008;32:872-6. [Crossref] [PubMed]
- Koolen PG, Schreinemacher MH, Peppelenbosch AG. Heterotopic ossifications in midline abdominal scars: a critical review of the literature. Eur J Vasc Endovasc Surg 2010;40:155-9. [Crossref] [PubMed]
- Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003;85:700-5. [PubMed]
- Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification revisited. Orthopedics 2011;34:177. [Crossref] [PubMed]
- White LM, Kandel R. Osteoid-producing tumors of bone. Semin Musculoskelet Radiol 2000;4:25-43. [Crossref] [PubMed]
- Huang TC, Monsour PA, Chahoud CD. Parosteal osteosarcoma: report of a case and review of the literature. Aust Dent J 2010;55:86-91. [Crossref] [PubMed]
- Resnick D, Niwayama G. Soft tissues. In: Resnick D, Niwayama G. editors. Diagnosisof Bone and Joint Disorders. 2nd ed. Philadelphia, PA: W.B. Saunders, 1988:4171-294.
- Soldatos T, McCarthy EF, Attar S, Carrino JA, Fayad LM. Imaging features of chondrosarcoma. J Comput Assist Tomogr 2011;35:504-11. [Crossref] [PubMed]
- Lameijer A, Murrmann GB, Apers JA. Bone in a laparotomy scar. Ned Tijdschr Geneeskd 2014;158:A7696. [PubMed]


