Magnetic resonance imaging of retroperitoneal interfascial plane involvement in acute pancreatitis
Introduction
Acute pancreatitis (AP) is a commonly seen severe abdominal disease. It can accompany spreading of inflammatory process and pancreatic fluid leakage (1). The mortality of mild AP is low (2); however 20% of patients can progress to severe pancreatitis. Acute severe pancreatitis patients can have a rapid disease onset, multiple organ dysfunction and failure, and a high fatality rate (3). Early diagnosis and evaluation of the severity of AP is important for making optimal treatment planning and thereafter reducing mortality.
The pancreas is located in the anterior pararenal space and lacks fascia on its surface. When pancreatitis occurs, inflammatory liquid can penetrate into the retroperitoneal, abdominal, and pelvic cavities (4). Traditionally, the retroperitoneal cavity is divided into three parts: the anterior pararenal space, perirenal space and posterior pararenal space. These are defined by the prerenal fascia, posterior renal fascia and lateroconal fascia (5). Molmenti et al. (6) proposed the interfascial planes as an anatomical concept regarding the retroperitoneal space, suggesting that each retroperitoneal fascia is composed of multiple discrete layers and the potential space between the apposed layers of fascia. The author divides the retroperitoneal space into nine parts: three spaces (anterior pararenal, perirenal and posterior pararenal spaces) and six interfascial planes (retromesenteric, retrorenal, lateroconal interfascial, and combined interfascial planes; presacral and prevesical spaces). Interfascial planes may better explain the spread of effusion in the retroperitoneal space during pancreatitis process. Ishikawa et al. (7) proposed that in addition there was also a subfascial plane between the posterior pararenal space and transversalis fascia, and that this plane was connected to the retrorenal plane through a narrow passage. The author studied the CT manifestations of AP involving interfascial planes, graded the spread of effusion between the interfascial planes, and found that classification of acute pancreatitis based on CT-determined retroperitoneal extension is a useful indicator of disease severity and prognosis.
In recent years, magnetic resonance imaging (MRI) has increasingly been used in the diagnosis of AP. CT is a convenient and rapid means to diagnose AP, but repeated CT follow-up exams pose the harm of accumulative X-ray radiation. MRI can accurately evaluate the necrosis and inflammation associated with AP and some scholars believe that it is superior to enhanced CT in predicting the severity of AP (8,9). MRI is able to identify early or mild AP that may be missed on CT, but without the radiation exposure (9). In addition, patients may experience adverse reaction to the iodinated contrast media. When iodinated contrast media is used for contrast-enhanced CT of AP, the micro-circulation may be negatively affected (10,11). MRI is the safest and most effective noninvasive imaging method to evaluate the pancreas and ductal system (12-15). MRI is also superior to other imaging techniques for the characterization of peripancreatic fluid collections (16,17).
The interfascial plane involvement in AP is commonly seen on MRI. We conducted this study to determine the characteristics of interfascial plane involvement of AP on MRI and to analyze the correlations of interfascial plane involvement with the magnetic resonance severity index (MRSI) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system.
Methods
Patient selection
This retrospective study was approved by our institutional review board (No. SKLMC-2013-012). Patient informed consent was waived. Patients with AP who were admitted to The Affiliated Hospital of North Sichuan Medical College, Nanchong, China, between November 2011 and August 2013 were candidates of this study. The diagnosis of AP was based on the presence of typical abdominal pain combined with three-fold elevated amylase or lipase. The inclusion criteria in this study were as follows: (I) pancreatitis at first onset; (II) abdominal MR examination performed; (III) inpatient; and (IV) three-fold elevated amylase or lipase, excluding other causes of elevated enzymes. The exclusion criteria were as follows: (I) history of chronic pancreatitis; (II) AP due to pancreatic carcinoma; and (III) emerging hypoproteinemia. These criteria have been described in our previous publications (18,19). In total consecutive 316 patients with AP were enrolled in this study, including 154 women and 162 men with a mean age of 53±15 years (range, 16–87 years).
MRI technique
All MR examinations were performed during suspended respiration with a 1.5-T system (Signa, GE Medical Systems, Milwaukee, WI, USA). The sequences included: Axial spoiled dual gradient-echo T1-weighted image (GRE T1WI), with the following scanning parameters: TR =170 ms, TE =2.7 ms, flip angle =80°, FOV =26–32 cm, section thickness =5–8 mm, and intersection gap =0.5–1.0 mm. Axial respiratory-triggered fast recovery fast spin-echo T2-weighted image (FRFSE T2WI) with fat suppression, with the following scanning parameters: TR =10,000–12,000 ms, TE =90–100 ms, FOV =34×34 cm2, section thickness =5 mm, and intersection gap =0.5 mm. Coronal, axial and sagittal single shot fast spin-echo T2-weighted image (SSFSE T2WI), with the following scanning parameters: TR =2,500–3,500 ms, TE =80–100 ms, FOV =34×34 cm2 section thickness =5 mm, and intersection gap =0.5 mm. SSFSE radial series slab MR cholangiopancreatography (MRCP) with the following scanning parameters: TR =6,000 ms, TE =830–1,100 ms, FOV =32–34 cm and section thickness =40–50 mm.
Axial slab three-dimensional (3D) spoiled gradient-echo (SPGR) dynamic contrast-enhanced MR imaging with fat suppression, with the following scanning parameters: TR =6.1 ms, TE =2.1 ms, flip angle =15°–20°, FOV =32–34 cm, and section thickness =5 mm. Three-dimensional SPGR was obtained at 2.5 mm increments with zero-fill interpolation for dynamic enhancement. Twenty milliliters of gadolinium (Magnevist; Schering Guangzhou Co., China) was administered intravenously with a pressure injector (Spectris MR Injection System, Medrad Inc, USA) at 2–3 mL/s and followed by a 20-mL saline solution flush. First-pass arterial enhancement was optimized with a timing bolus sequence (axial FMPSPGR). Dynamic imaging was performed during breath-holding before the injection (unenhanced), immediately after the injection (hepatic arterial phase), 30 seconds after the injection (early venous phase), and 1 min after the injection (late venous phase). The delayed phases were acquired with axial fast spoiled gradient echo (FSPGR) and another 3D SPGR T1-weighted sequence.
MRI image interpretation
All the original MRI data were loaded onto a GE healthcare workstation (version: AW 4.4) for reading and processing. Two radiologists who had more than 4 years of experience interpreting abdominal MR images reviewed the MRI images. They were both blinded to the clinical outcomes and laboratory data. The review included the MRI manifestation and severity of the interfascial plane involvement in AP and the MRSI grading. Any discrepancy between the two observers was resolved by consensus discussion. According to the MRSI guidelines, AP was divided into mild (0–3 points), moderate (4–6 points) and severe (7–10 points) (20).
APACHE II is widely used clinically to score AP severity (21). The patients’ physiology and laboratory examination indices were collected to grade the APACHE II; these factors included age, body temperature, mean arterial pressure, heart rate, arterial partial pressure of oxygen, arterial PH, serum potassium concentration, serum sodium concentrations, serum creatinine, peripheral white blood cells, hematocrit, previous health conditions and Glasgow coma scale (22). According to the APACHE II scoring system, AP was graded as mild (<8 points) or severe AP (≥8 points) (23). Two physicians calculated the scores according to the clinical outcomes and laboratory data.
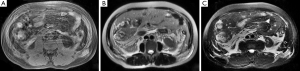
The involvement of the retromesenteric plane (Figure 1), lateroconal plane, retrorenal plane, combined interfascial plane, subfascial plane, anterior pararenal space, perirenal space and posterior pararenal space was recorded (6,7). The interfascial plane involvement was defined as interfascial plane edema, thickening (diameter>3 mm) and interfascial plane effusion (7,24), which showed isointensity or hypointensity on T1WI, hyperintensity on T2WI with fat suppression. The anterior pararenal space, perirenal space and posterior pararenal space involvement appeared as linear or patchy high signal on T2WI and T2WI with fat suppression, and isointensity or low signal on T1WI.
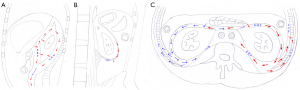
With reference to CT classification of the effusion spreading between interfascial planes and the different rates of interfascial plane involvement (7), in this study the severity of the interfascial plane involvement in AP on MRI was classified into six grades: grade 0, normal retroperitoneal interfascial planes and perirenal space (recorded as 0 point); grade 1, inflammation confined to the anterior pararenal space or retromesenteric plane (recorded as 1 point); grade 2, inflammation spreading into the lateroconal plane or retrorenal plane (recorded as 2 points); grade 3, inflammation spreading into the combined interfascial plane (recorded as 3 points); grade 4, inflammation spreading into the subfascial plane (recorded as 4 points); and grade 5, inflammation intruding into the posterior pararenal space (recorded as 5 points).
Results
Of the 316 patients with AP, the etiology of AP was biliary in 165 (52.2%) cases, hyperlipidemia-related in 56 (17.7%) cases, alcohol-related in 19 (6.0%) cases, pregnancy-related in 5 (1.6%) cases, over-eating-related in 9 (2.8%) cases, and unknown in 62 (19.6%) cases. The mean APACHE-II score was 4.7±3.4 points (ranging from 0 to 21 points). A total of 263 patients had mild AP (<8 points) with an average APACHE-II score of 2.2±1.3 points, while 47 patients had severe AP (≥8 points) with an APACHE-II score of 2.9±1.1 points.
A total of 110 AP patients (34.8%) had both plain scans and dynamic enhanced MR imaging, while 206 (65.2%) had only plain scans. The agreement between the observers for MRSI was good (κ=0.788). According to MRSI, 42.1% (133/316), 50.9% (161/316) and 7.0% (22/316) of patients had mild, moderate and severe AP, respectively, with a mean MRSI score of 3.9±1.7. Of the 316 patients with AP, 244 were diagnosed with edematous AP, while 72 patients were diagnosed with necrotizing AP on MRI. Among the 72 patients with necrotizing AP, 50 had necrosis of less than 30% of the total pancreatic volume, 21 had necrosis of 30% to 50%, and 1 had necrosis of more than 50%.
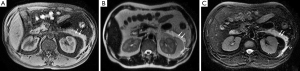
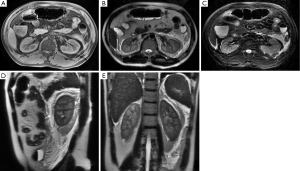
The agreement between the observers for evaluating interfascial plane involvement was had a κ=0.765. In the 316 patients with AP, 92.7% (293/316) had interfascial plane involvement, including 245 patients with interfascial plane edema and thickening and 36 patients with interfascial plane effusion (Table 1). The interfascial plane involvement score on MRI was 2.3±1.3 (ranging from 0 to 5). The severity of the interfascial plane involvement in AP on MRI was showed as follows: the number of grade 0 were 23, grade 1 were 60, grade 2 were 105 (Figure 2), grade 3 were 78 (Figure 3), grade 4 were 25 and grade 5 were 25 (Figure 4). The specific details of interfascial plane involvement in AP on MRI are listed in Table 2.
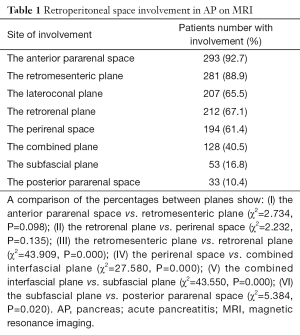
Full table
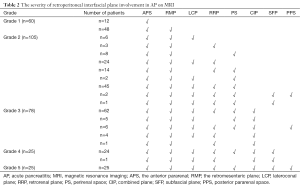
Full table
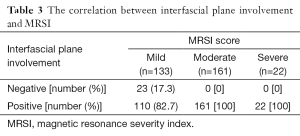
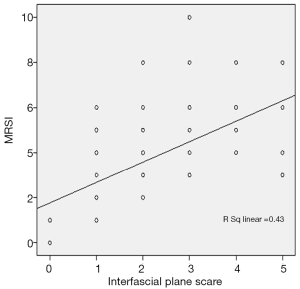
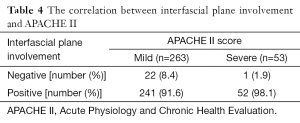
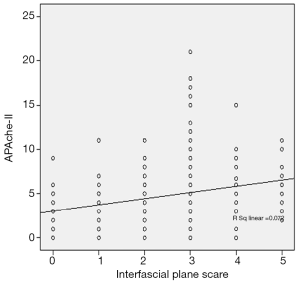
According to the MRSI score, the prevalence of interfascial plane involvement in mild, moderate and severe AP is listed in Table 3. The interfascial plane involvement on MRI was strongly correlated with the MRSI score (r=0.703) (Figure 5). According to the APACHE-II score, the prevalence of the interfascial plane involvement in mild and severe AP is listed in Table 4. The interfascial plane involvement on MRI was only weakly correlated with the APACHE-II score (r=0.291) (Figure 6).
Full table
Full table
Discussion
MRI can detect interfascial plane involvement in AP and show the pathway of the inflammatory spread along the interfascial planes in different severities of AP, which may be valuable in determining the severity of AP. In this study, we found that 92.7% of patients with AP had interfascial plane involvement on MRI. The interfascial plane involvement on MRI was strongly correlated with the MRSI score (r=0.703), and weakly correlated with the APACHE-II score (r=0.291). Compared the concept of interfascial plane with the traditional view, it could better explain the pathological process of the spread and the scope in the retroperitoneal space. It is of great importance in image positioning, effusion drainaging and surgical treatment. Ishikawa et al. (7) studied the CT manifestations of AP involving interfascial planes, graded the spread of effusion among the interfascial planes and analyzed the relationship between the severity of interfascial plane involvement and the severity of AP. However, it has yet to be seen the study of interfascial plane involvement using MR and the relationship with the severity of AP. MRI has increasingly been used in the diagnosis of AP. MRI can accurately identify interfascial plane involvement in AP because it is sensitive to the fluid patterns present (25,26). T2-weighted fat-suppressed images are sensitive in the detection of subtle early pancreatitis in patients who have negative CT scan findings (9). In our study, 82.7%, 100% and 100% of patients had interfascial plane involvement in mild, moderate and severe AP, according to MRSI, respectively. According to the frequency of interfascial plane involvement on MRI, we speculate that the pathway by which inflammation spread was as follows: inflammation first spread to the anterior pararenal space and retromesenteric plane, then along the interfascial plane into the lateroconal plane and retrorenal plane or break through fascia into the perirenal space, next into the combined interfascial plane and finally spread to the subfascial plane and posterior pararenal space.
The perirenal fascia was made up of a single multilaminated structure with potential space, and the concept of interfascial planes can better explain the spread of the effusion in the retroperitoneal space (6,27). This study identified the involvement of each interfascial plane in different degrees of AP; we hypothesize the pathway of inflammation spread and the pathway involvement order and divide the involvement into six grades. The inflammatory substances produced in AP spread in the retroperitoneum in two ways: spreading through the communicating anatomic spaces or breaking directly through the peritoneum structure. When AP occurs, the inflammatory substances first dissolve and damage the renal fascia and then spread to the retromesenteric plane and then the retrorenal plane, lateroconal interfascial plane and the contralateral retromesenteric plane with which it connected. The inflammation can spread from the retrorenal plane to the subfascial plane through a narrow passage. After the renal fascia dissolves, the inflammation can spread to the perirenal space and posterior pararenal space (28,29). It can also spread to the combined interfascial plane under the kidney along the retromesenteric plane and retrorenal plane, then to the presacral space and prevesical space in the pelvic cavity. When the subfascial plane is involved, AP can cause the Grey-Turner sign (30). Clinically, skin changes in AP patients predict worsening anemia and a high mortality rate. The ability to observe the degree of interfascial plane involvement may potentially aid in predicting the course of the disease.
MRSI reflect local complications of AP, and interfascial plane involvement was strongly correlated with the MRSI score (r=0.703). When the severity of AP increases, the frequency of interfascial plane involvement also increases. The APACHE II reflects systemic complications of AP. The higher the APACHE II score, the worse the patient’s general condition (31). In this study, the mean APACHE-II scores of mild and severe AP were 2.2±1.3 and 2.9±1.1 points, respectively. However, the interfascial plane involvement on MRI was only weakly correlated with the APACHE-II score (r=0.291). The interfascial plane involvement for forecasting systemic complications in AP patients remain to be further studied. Probably further analysis of subgroup involvement can provide further insight.
In conclusion, interfascial plane involvement in AP is common on MRI. MRI accurately depicts the involvement of interfascial planes and the pathway of inflammation spread in AP of various severities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the authors’ institutional review board (No. SKLMC-2013-012) and written informed consent was obtained from all patients.
References
- Schepers NJ, Besselink MG, van Santvoort HC, Bakker OJ, Bruno MJ; Dutch Pancreatitis Study Group. Early management of acute pancreatitis. Best Pract Res Clin Gastroenterol 2013;27:727-43. [Crossref] [PubMed]
- Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, Shaheen NJ, Sandler RS. Digestive and liver diseases statistics, 2004. Gastroenterology 2004;126:1448-53. [Crossref] [PubMed]
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379-400. [Crossref] [PubMed]
- Mortelé KJ, Mergo PJ, Taylor HM, Ernst MD, Ros PR. Renal and perirenal space involvement in acute pancreatitis: spiral CT findings. Abdom Imaging 2000;25:272-8. [Crossref] [PubMed]
- Meyers MA. The extraperitoneal spaces: normal and pathologic anatomy. In: Meyers MA, editor. Dynamic radiology of the abdomen: normal and pathologic anatomy. New York: Springer-Verlag, 2000:333-492.
- Molmenti EP, Balfe DM, Kanterman RY, Bennett HF. Anatomy of the retroperitoneum: observations of the distribution of pathologic fluid collections. Radiology 1996;200:95-103. [Crossref] [PubMed]
- Ishikawa K, Idoguchi K, Tanaka H, Tohma Y, Ukai I, Watanabe H, Matsuoka T, Yokota J, Sugimoto T. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol 2006;60:445-52. [Crossref] [PubMed]
- Kim YK, Kim CS, Han YM. Role of fat-suppressed t1-weighted magnetic resonance imaging in predicting severity and prognosis of acute pancreatitis: an intraindividual comparison with multidetector computed tomography. J Comput Assist Tomogr 2009;33:651-6. [Crossref] [PubMed]
- Bollen TL. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin North Am 2012;50:429-45. [Crossref] [PubMed]
- Wang YX, Chen S, Morcos SK. Contrast-enhanced CT in acute pancreatitis. Br J Radiol 1999;72:1029. [Crossref] [PubMed]
- Schmidt J, Hotz HG, Foitzik T, Ryschich E, Buhr HJ, Warshaw AL, Herfarth C, Klar E. Intravenous contrast medium aggravates the impairment of pancreatic microcirculation in necrotizing pancreatitis in the rat. Ann Surg 1995;221:257-64. [Crossref] [PubMed]
- Drake LM, Anis M, Lawrence C. Accuracy of magnetic resonance cholangiopancreatography in identifying pancreatic duct disruption. J Clin Gastroenterol 2012;46:696-9. [Crossref] [PubMed]
- Liu N, Huang XH, Zhang XM, Dong GL, Jing ZL, Gao CL, Tang MY. The angle of pancreaticobiliary junction correlates with acute pancreatitis: a magnetic resonance cholangiopancreatography study. Quant Imaging Med Surg 2015;5:401-6. [PubMed]
- Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. Acute pancreatitis successfully diagnosed by diffusion-weighted imaging: a case report. World J Gastroenterol 2008;14:5478-80. [Crossref] [PubMed]
- Manikkavasakar S, AlObaidy M, Busireddy KK, Ramalho M, Nilmini V, Alagiyawanna M, Semelka RC. Magnetic resonance imaging of pancreatitis: an update. World J Gastroenterol 2014;20:14760-77. [Crossref] [PubMed]
- Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology 1999;211:727-35. [Crossref] [PubMed]
- Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology 1997;203:773-8. [Crossref] [PubMed]
- Chi XX, Zhang XM, Chen TW, Huang XH, Yang L, Tang W, Xiao B. The normal transverse mesocolon and involvement of the mesocolon in acute pancreatitis: an MRI study. PLoS One 2014;9:e93687. [Crossref] [PubMed]
- Chi XX, Zhang XM, Chen TW, Tang W, Xiao B, Ji YF, Huang XH. Magnetic resonance imaging for the normal mesostenium and involvement of the mesostenium in acute pancreatitis. Biomed Res Int 2014;2014:924845.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS, Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-90. [Crossref] [PubMed]
- Raptopoulos V, Kleinman PK, Marks S Jr, Snyder M, Silverman PM. Renal fascial pathway: posterior extension of pancreatic effusions within the anterior pararenal space. Radiology 1986;158:367-74. [Crossref] [PubMed]
- Semelka RC, Kroeker MA, Shoenut JP, Kroeker R, Yaffe CS, Micflikier AB. Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology 1991;181:785-91. [Crossref] [PubMed]
- Chilla GS, Tan CH, Xu C, Poh CL. Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant Imaging Med Surg 2015;5:407-22. [PubMed]
- Dodds WJ, Darweesh RM, Lawson TL, Stewart ET, Foley WD, Kishk SM, Hollwarth M. The retroperitoneal spaces revisited. AJR Am J Roentgenol 1986;147:1155-61. [Crossref] [PubMed]
- Hashimoto M, Okane K, Hirano H, Watarai J. Pictorial review: Subperitoneal spaces of the broad ligament and sigmoid mesocolon--imaging findings. Clin Radiol 1998;53:875-81. [Crossref] [PubMed]
- Tang MY, Chen TW, Huang XH, Li XH, Wang SY, Liu N, Zhang XM. Acute pancreatitis with gradient echo T2*-weighted magnetic resonance imaging. Quant Imaging Med Surg 2016;6:157-67. [Crossref] [PubMed]
- Sugimoto M, Takada T, Yasuda H, Nagashima I, Amano H, Yoshida M, Miura F, Uchida T, Isaka T, Toyota N, Wada K, Takagi K, Kato K, Takeshita K. MPR-hCT imaging of the pancreatic fluid pathway to Grey-Turner's and Cullen's sign in acute pancreatitis. Hepatogastroenterology. 2005;52:1613-6. [PubMed]
- De Campos T, Cerqueira C, Kuryura L, Parreira JG, Soldá S, Perlingeiro JA, Assef JC, Rasslan S. Morbimortality indicators in severe acute pancreatitis. JOP 2008;9:690-7. [PubMed]


